Interim estimates of 2013-14 seasonal influenza vaccine effectiveness - United States, February 2014
- PMID: 24553196
- PMCID: PMC4584757
Interim estimates of 2013-14 seasonal influenza vaccine effectiveness - United States, February 2014
Abstract
In the United States, annual vaccination against seasonal influenza is recommended for all persons aged ≥6 months. Each season since 2004-05, CDC has estimated the effectiveness of seasonal influenza vaccine to prevent influenza-associated, medically attended acute respiratory illness (ARI). This report uses data from 2,319 children and adults enrolled in the U.S. Influenza Vaccine Effectiveness (Flu VE) Network during December 2, 2013-January 23, 2014, to estimate an interim adjusted effectiveness of seasonal influenza vaccine for preventing laboratory-confirmed influenza virus infection associated with medically attended ARI. During this period, overall vaccine effectiveness (VE) (adjusted for study site, age, sex, race/ethnicity, self-rated health, and days from illness onset to enrollment) against influenza A and B virus infection associated with medically attended ARI was 61%. The influenza A (H1N1)pdm09 (pH1N1) virus that emerged to cause a pandemic in 2009 accounted for 98% of influenza viruses detected. VE was estimated to be 62% against pH1N1 virus infections and was similar across age groups. As of February 8, 2014, influenza activity remained elevated in the United States, the proportion of persons seeing their health-care provider for influenza-like illness was lower than in early January but remained above the national baseline, and activity still might be increasing in some parts of the country. CDC and the Advisory Committee on Immunization Practices routinely recommend that annual influenza vaccination efforts continue as long as influenza viruses are circulating. Persons aged ≥6 months who have not yet been vaccinated this season should be vaccinated. Antiviral medications are an important second line of defense to treat influenza illness and should be used as recommended among suspected or confirmed influenza patients, regardless of patient vaccination status. Early antiviral treatment is recommended for persons with suspected influenza with severe or progressive illness (e.g., hospitalized persons) and those at high risk for complications from influenza, no matter how severe the illness.
Figures
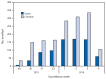
References
-
- CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2012–13 influenza season. MMWR. 2012;61:613–8. - PubMed
-
- CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2011;60(RR-1) - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

