Recent advances in oral vaccine development: yeast-derived β-glucan particles
- PMID: 24553259
- PMCID: PMC4896516
- DOI: 10.4161/hv.28166
Recent advances in oral vaccine development: yeast-derived β-glucan particles
Abstract
Oral vaccination is the most challenging vaccination method due to the administration route. However, oral vaccination has socio-economic benefits and provides the possibility of stimulating both humoral and cellular immune responses at systemic and mucosal sites. Despite the advantages of oral vaccination, only a limited number of oral vaccines are currently approved for human use. During the last decade, extensive research regarding antigen-based oral vaccination methods have improved immunogenicity and induced desired immunological outcomes. Nevertheless, several factors such as the harsh gastro-intestinal environment and oral tolerance impede the clinical application of oral delivery systems. To date, human clinical trials investigating the efficacy of these systems are still lacking. This review addresses the rationale and key biological and physicochemical aspects of oral vaccine design and highlights the use of yeast-derived β-glucan microparticles as an oral vaccine delivery platform.
Keywords: GALT; Peyer’s patches; antigen delivery vehicles; microparticles; oral; vaccination; yeast-derived beta-glucan.
Figures
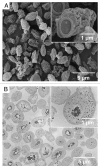
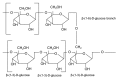
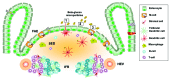
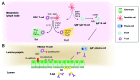
References
-
- World Health Organization. Diarrhoeal disease, fact sheet number 330. 2013. Available from: http://www.who.int/mediacentre/factsheets/fs330/en/
-
- Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, et al. , Child Health Epidemiology Reference Group of WHO and UNICEF. . Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 2010; 375:1969 - 87; http://dx.doi.org/10.1016/S0140-6736(10)60549-1; PMID: 20466419 - DOI - PubMed
-
- Levine MM. . Immunization against bacterial diseases of the intestine. J Pediatr Gastroenterol Nutr 2000; 31:336 - 55; http://dx.doi.org/10.1097/00005176-200010000-00003; PMID: 11045827 - DOI - PubMed
-
- Brandtzaeg P. . Induction of secretory immunity and memory at mucosal surfaces. Vaccine 2007; 25:5467 - 84; http://dx.doi.org/10.1016/j.vaccine.2006.12.001; PMID: 17227687 - DOI - PubMed
-
- Holmgren J, Czerkinsky C. . Mucosal immunity and vaccines. Nat Med 2005; 11:Suppl S45 - 53; http://dx.doi.org/10.1038/nm1213; PMID: 15812489 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
