Use of vaccines as probes to define disease burden
- PMID: 24553294
- PMCID: PMC4682543
- DOI: 10.1016/S0140-6736(13)61682-7
Use of vaccines as probes to define disease burden
Erratum in
- Lancet. 2014 Jun 21;383(9935):2126
Abstract
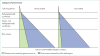
Vaccine probe studies have emerged in the past 15 years as a useful way to characterise disease. By contrast, traditional studies of vaccines focus on defining the vaccine effectiveness or efficacy. The underlying basis for the vaccine probe approach is that the difference in disease burden between vaccinated and unvaccinated individuals can be ascribed to the vaccine-specific pathogen. Vaccine probe studies can increase understanding of a vaccine's public health value. For instance, even when a vaccine has a seemingly low efficacy, a high baseline disease incidence can lead to a large vaccine-preventable disease burden and thus that population-based vaccine introduction would be justified. So far, vaccines have been used as probes to characterise disease syndromes caused by Haemophilus influenzae type b, pneumococcus, rotavirus, and early infant influenza. However, vaccine probe studies have enormous potential and could be used more widely in epidemiology, for example, to define the vaccine-preventable burden of malaria, typhoid, paediatric influenza, and dengue, and to identify causal interactions between different pathogens.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
DRF has no conflicts to declare. BDG works for AMP, which receives unrestricted support from Sanofi-Aventis and grant-specific support from Crucell, GlaxoSmithKline, Merck, Novartis, Pfizer, and Sanofi-Aventis. JAGS has received research funding from GlaxoSmithKline Biologicals and travel expenses from Merck.
Figures
References
-
- Mulholland EK. Use of vaccine trials to estimate burden of disease. J Health Popul Nutr. 2004;22:257–67. - PubMed
-
- Mulholland K, Levine O, Nohynek H, Greenwood BM. Evaluation of vaccines for the prevention of pneumonia in children in developing countries. Epidemiol Rev. 1999;21:43–55. - PubMed
-
- Bale JR. Creation of a research program to determine the etiology and epidemiology of acute respiratory tract infection among children in developing countries. Rev Infect Dis. 1990;12(suppl 8):S861–66. - PubMed
-
- Mulholland K, Hilton S, Adegbola R, et al. Randomised trial of Haemophilus influenzae type-b tetanus protein conjugate vaccine [corrected] for prevention of pneumonia and meningitis in Gambian infants. Lancet. 1997;349:1191–97. - PubMed
-
- Bennett JV, Platonov AE, Slack MP, Mala P, Burton AH, Robertson SE. Haemophilus influenzae type b (Hib) meningitis in the pre-vaccine era: a global review of incidence, age distribution, and case-fatality rates. Geneva: World Health Organization; 2002.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

