Unique macrophages different from M1/M2 macrophages inhibit T cell mitogenesis while upregulating Th17 polarization
- PMID: 24553452
- PMCID: PMC3930092
- DOI: 10.1038/srep04146
Unique macrophages different from M1/M2 macrophages inhibit T cell mitogenesis while upregulating Th17 polarization
Abstract
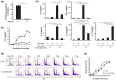
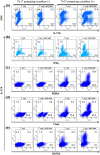
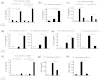
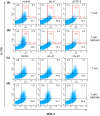
Mycobacterial infection induces suppressor macrophages (MΦs), causing disease exacerbation. There are two major MΦ subsets (M1 and M2 MΦs) that are phenotypically and functionally different. Here, we examined which of the MΦ subsets the mycobacterial infection-induced suppressor MΦs (MIS-MΦs) belong to. MIS-MΦs down-regulated T cell production of Th1 and Th2 cytokines but markedly increased production of interleukin (IL)-17A and IL-22 through up-regulation of Th17 cell expansion. In this phenomenon, a novel MΦ population, which is functionally distinguishable from M1 and M2 MΦ subsets and possesses unique phenotypes (IL-12(+), IL-1β(high), IL-6(+), tumor necrosis factor (TNF)-α(+), nitric oxide synthase (NOS) 2(+), CCR7(high), IL-10(high), arginase (Arg)-1(-), mannose receptor (MR)(low), Ym1(high), Fizz(low), and CD163(high)), played central roles through the action of IL-6 and transforming growth factor (TGF)-β but not IL-21 and IL-23. This new type of MΦ population was induced in infected mice and actively supported the in vivo expansion of Th17 cells.
Figures

References
-
- Benoit M., Desnues B. & Mege J. L. Macrophage polarization in bacterial infections. J. Immunol. 181, 3733–3739 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

