Differential entrainment and learning-related dynamics of spike and local field potential activity in the sensorimotor and associative striatum
- PMID: 24553926
- PMCID: PMC3931500
- DOI: 10.1523/JNEUROSCI.1782-13.2014
Differential entrainment and learning-related dynamics of spike and local field potential activity in the sensorimotor and associative striatum
Abstract
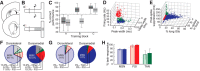
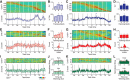
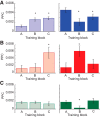
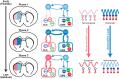
Parallel cortico-basal ganglia loops are thought to have distinct but interacting functions in motor learning and habit formation. In rats, the striatal projection neuron populations (MSNs) in the dorsolateral and dorsomedial striatum, respectively corresponding to sensorimotor and associative regions of the striatum, exhibit contrasting dynamics as rats acquire T-maze tasks (Thorn et al., 2010). Here, we asked whether these patterns could be related to the activity of local interneuron populations in the striatum and to the local field potential activity recorded simultaneously in the corresponding regions. We found that dorsolateral and dorsomedial striatal fast-spiking interneurons exhibited task-specific and training-related dynamics consistent with those of corresponding MSN populations. Moreover, both MSNs and interneuron populations in both regions became entrained to theta-band (5-12 Hz) frequencies during task acquisition. However, the predominant entrainment frequencies were different for the sensorimotor and associative zones. Dorsolateral striatal neurons became entrained mid-task to oscillations centered ∼ 5 Hz, whereas simultaneously recorded neurons in the dorsomedial region became entrained to higher frequency (∼ 10 Hz) rhythms. These region-specific patterns of entrainment evolved dynamically with the development of region-specific patterns of interneuron and MSN activity, indicating that, with learning, these two striatal regions can develop different frequency-modulated circuit activities in parallel. We suggest that such differential entrainment of sensorimotor and associative neuronal populations, acquired through learning, could be critical for coordinating information flow throughout each trans-striatal network while simultaneously enabling nearby components of the separate networks to operate independently.
Keywords: dorsolateral; dorsomedial; electrophysiology; habit learning; oscillation; striatum.
Figures




References
-
- Aosaki T, Kimura M, Graybiel AM. Temporal and spatial characteristics of tonically active neurons of the primate's striatum. J Neurophysiol. 1995;73:1234–1252. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
