Spatial characterization of hot melt extruded dispersion systems using thermal atomic force microscopy methods: the effects of processing parameters on phase separation
- PMID: 24554116
- PMCID: PMC4062809
- DOI: 10.1007/s11095-013-1279-x
Spatial characterization of hot melt extruded dispersion systems using thermal atomic force microscopy methods: the effects of processing parameters on phase separation
Abstract
Purpose: In this study we explore the use of nano-scale localized thermal analysis (LTA) and transition temperature microcopy (TTM) as a novel combined approach to studying phase separation in HME dispersions of cyclosporine A in Eudragit EPO.
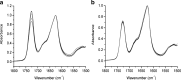
Methods: Modulated temperature differential scanning calorimetry (MTDSC), attenuated total reflectance FTIR spectroscopy, nano-LTA and TTM were performed on raw materials and dispersions prepared by hot melt extrusion (HME) and spin coating. For samples prepared by HME, two mixing temperatures (110°C and 150°C) and residence times (5 and 15 min) were investigated.
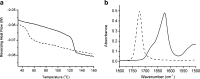
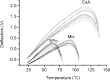
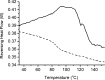
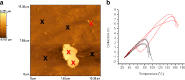
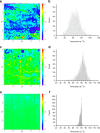
Results: Spin coated samples showed an intermediate T g for the mixed systems consistent with molecular dispersion formation. The HME samples prepared at 110°C showed evidence of inhomogeneity using MTDSC and FTIR, while those produced at 150°C h showed evidence for the formation of a single phase system using MTDSC. The nanothermal methods, however, indicated the presence of phase separated cyclosporine A at the higher preparation temperature while the TTM was able to map regions of differing penetration temperatures, indicating the presence of compositionally inhomogeneous regions in all but the high processing temperature/high residence time samples.
Conclusions: TTM is a potentially important new method for studying phase separation and that such separation may remain undetected or poorly understood using conventional bulk analytical techniques.
Figures
Similar articles
-
Hot melt extrusion (HME) for amorphous solid dispersions: predictive tools for processing and impact of drug-polymer interactions on supersaturation.Eur J Pharm Sci. 2013 Feb 14;48(3):371-84. doi: 10.1016/j.ejps.2012.12.012. Epub 2012 Dec 23. Eur J Pharm Sci. 2013. PMID: 23267847
-
Characterisation and prediction of phase separation in hot-melt extruded solid dispersions: a thermal, microscopic and NMR relaxometry study.Pharm Res. 2010 Sep;27(9):1869-83. doi: 10.1007/s11095-010-0185-8. Epub 2010 Jun 29. Pharm Res. 2010. PMID: 20585845
-
Compositional analysis of low quantities of phase separation in hot-melt-extruded solid dispersions: a combined atomic force microscopy, photothermal fourier-transform infrared microspectroscopy, and localised thermal analysis approach.Pharm Res. 2011 Sep;28(9):2311-26. doi: 10.1007/s11095-011-0461-2. Epub 2011 May 24. Pharm Res. 2011. PMID: 21607778
-
A New Extrudable Form of Hypromellose: AFFINISOL™ HPMC HME.AAPS PharmSciTech. 2016 Feb;17(1):106-19. doi: 10.1208/s12249-015-0395-9. Epub 2015 Sep 4. AAPS PharmSciTech. 2016. PMID: 26335416 Free PMC article.
-
Optimising Drug Solubilisation in Amorphous Polymer Dispersions: Rational Selection of Hot-melt Extrusion Processing Parameters.AAPS PharmSciTech. 2016 Feb;17(1):200-13. doi: 10.1208/s12249-015-0450-6. Epub 2016 Jan 4. AAPS PharmSciTech. 2016. PMID: 26729536 Free PMC article.
Cited by
-
Microstructure Formation and Characterization of Long-Acting Injectable Microspheres: The Gateway to Fully Controlled Drug Release Pattern.Int J Nanomedicine. 2024 Feb 19;19:1571-1595. doi: 10.2147/IJN.S445269. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38406600 Free PMC article. Review.
-
Physicochemical and prebiotic properties of resistant starch from Musa sapientum Linn., ABB group, cv. Kluai Namwa Luang.Heliyon. 2020 Dec 21;6(12):e05789. doi: 10.1016/j.heliyon.2020.e05789. eCollection 2020 Dec. Heliyon. 2020. PMID: 33376829 Free PMC article.
References
-
- Djuris J, Nikolakakis I, Ibric S, Djuric Z, Kachrimanis K. Preparation of carbamazepine-Soluplus (R) solid dispersions by hot-melt extrusion, and prediction of drug-polymer miscibility by thermodynamic model fitting. Eur J Pharm Biopharm. 2013;84(1):228–237. doi: 10.1016/j.ejpb.2012.12.018. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

