STARR with CONTOUR® TRANSTAR™ device for obstructed defecation syndrome: one-year real-world outcomes of the European TRANSTAR registry
- PMID: 24554148
- PMCID: PMC3996277
- DOI: 10.1007/s00384-014-1836-8
STARR with CONTOUR® TRANSTAR™ device for obstructed defecation syndrome: one-year real-world outcomes of the European TRANSTAR registry
Abstract
Purpose: Stapled transanal rectal resection (STARR) in patients with obstructive defecation syndrome (ODS) is limited by the capacity of the circular stapler used. This prospective cohort study was conducted to assess real-world clinical outcomes of STARR with the new CONTOUR® TRANSTAR™ device, shortly named TRANSTAR, at 12 months postoperatively.
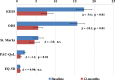
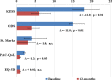
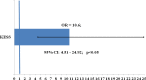
Methods: From January 2009 to January 2011, consecutive patients who underwent TRANSTAR in 22 European colorectal centers were enrolled in the study. Functional outcomes and quality of life were assessed by the changes in a number of scoring systems (Knowles-Eccersley-Scott-Symptom (KESS) score, ODS score, St. Mark's score, Euro Quality of Life-5 Dimension (EQ-5D) score, and Patient Assessment of Constipation-Quality of Life (PAC-QoL) score), at 12 months as compared to baseline. All complications were recorded and analyzed.
Results: A total of 100 patients (98% female), mean age 60 years, were entered in the study. Statistically significant improvements were seen in the KESS (median 18 vs. 6; p < 0.01), ODS (median 15 vs. 4; p < 0.01), and PAC-Qol scores (median 2.10 vs. 0.86; p < 0.01). St. Mark's and EQ-5D scores improved nonsignificantly. Complications were reported in 11 % of patients, including bleeding (5%), staple line complications (3%), urinary retention (2%), and persistent pain (1%). No major complications or mortality occurred.
Conclusion: TRANSTAR facilitated a tailored, real circumferential full-thickness rectal resection, leading to improved patient functional and quality of life outcomes at 12 months postoperatively. It represents a safe and effective treatment for ODS in local clinical practice, although the sustainability of real-world results needs to be proven in the long-term follow-up.
Figures

References
-
- Jayne D, Stuto A (Eds) (2009) Transanal stapling techniques for anorectal prolapse. London, Springer-Verlag London Limited. ISBN 978-1-84800-904-2
-
- Schwandner O, Farke S, Bruch HP. Stapled transanal rectal resection (STARR) for obstructed defection caused by rectocele and rectoanal intussusception. Viszeralchirurgie. 2005;40:331–341. doi: 10.1055/s-2005-872460. - DOI
-
- National Institute for Health and Clinical Excellence . Interventional procedure guidance 169: stapled transanal rectal resection for obstructed defaecation syndrome. London: National Institute for Health and Clinical Excellence; 2006.
-
- National Institute for Health and Clinical Excellence . Interventional procedure guidance 351: stapled transanal rectal resection for obstructed defaecation syndrome. London: National Institute for Health and Clinical Excellence; 2010.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

