VSV-GP: a potent viral vaccine vector that boosts the immune response upon repeated applications
- PMID: 24554655
- PMCID: PMC3993835
- DOI: 10.1128/JVI.03276-13
VSV-GP: a potent viral vaccine vector that boosts the immune response upon repeated applications
Abstract
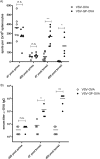
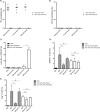
Antivector immunity limits the response to homologous boosting for viral vector vaccines. Here, we describe a new, potent vaccine vector based on replication-competent vesicular stomatitis virus pseudotyped with the glycoprotein of the lymphocytic choriomeningitis virus (VSV-GP), which we previously showed to be safe in mice. In mice, VSV and VSV-GP encoding ovalbumin (OVA) as a model antigen (VSV-OVA and VSV-GP-OVA) induced equal levels of OVA-specific humoral and cellular immune responses upon a single immunization. However, boosting with the same vector was possible only for VSV-GP-OVA as neutralizing antibodies to VSV limited the immunogenicity of the VSV-OVA boost. OVA-specific cytotoxic T-lymphocyte (CTL) responses induced by VSV-GP-OVA were at least as potent as those induced by an adenoviral state-of-the-art vaccine vector and completely protected mice in a Listeria monocytogenes challenge model. VSV-GP is so far the only replication-competent vaccine vector that does not lose efficacy upon repeated application.
Importance: Although there has been great progress in treatment and prevention of infectious diseases in the past several years, effective vaccines against some of the most serious infections, e.g., AIDS, malaria, hepatitis C, or tuberculosis, are urgently needed. Here, several approaches based on viral vector vaccines are under development. However, for all viral vaccine vectors currently in clinical testing, repeated application is limited by neutralizing antibodies to the vector itself. Here, we have exploited the potential of vesicular stomatitis virus pseudotyped with the glycoprotein of the lymphocytic choriomeningitis virus (VSV-GP) as a vaccine platform. VSV-GP is the first replication-competent viral vector vaccine that does not induce vector-specific humoral immunity, i.e., neutralizing antibodies, and therefore can boost immune responses against a foreign antigen by repeated applications. The vector allows introduction of various antigens and therefore can serve as a platform technology for the development of novel vaccines against a broad spectrum of diseases.
Figures




References
-
- Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, Truitt DM, Sumida SM, Kishko MG, Arthur JC, Korioth-Schmitz B, Newberg MH, Gorgone DA, Lifton MA, Panicali DL, Nabel GJ, Letvin NL, Goudsmit J. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172:6290–6297 - PubMed
-
- Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, Tang A, Chen M, Huang L, Harris V, Freed DC, Wilson KA, Dubey S, Zhu DM, Nawrocki D, Mach H, Troutman R, Isopi L, Williams D, Hurni W, Xu Z, Smith JG, Wang S, Liu X, Guan L, Long R, Trigona W, Heidecker GJ, Perry HC, Persaud N, Toner TJ, Su Q, Liang X, Youil R, Chastain M, Bett AJ, Volkin DB, Emini EA, Shiver JW. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305–6313. 10.1128/JVI.77.11.6305-6313.2003 - DOI - PMC - PubMed
-
- Santra S, Sun Y, Parvani JG, Philippon V, Wyand MS, Manson K, Gomez-Yafal A, Mazzara G, Panicali D, Markham PD, Montefiori DC, Letvin NL. 2007. Heterologous prime/boost immunization of rhesus monkeys by using diverse poxvirus vectors. J. Virol. 81:8563–8570. 10.1128/JVI.00744-07 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

