Hepatitis A virus adaptation to cellular shutoff is driven by dynamic adjustments of codon usage and results in the selection of populations with altered capsids
- PMID: 24554668
- PMCID: PMC3993836
- DOI: 10.1128/JVI.00087-14
Hepatitis A virus adaptation to cellular shutoff is driven by dynamic adjustments of codon usage and results in the selection of populations with altered capsids
Abstract
Hepatitis A virus (HAV) has a highly biased and deoptimized codon usage compared to the host cell and fails to inhibit host protein synthesis. It has been proposed that an optimal combination of abundant and rare codons controls the translation speed required for the correct capsid folding. The artificial shutoff host protein synthesis results in the selection of variants containing mutations in the HAV capsid coding region critical for folding, stability, and function. Here, we show that these capsid mutations resulted in changes in their antigenicity; in a reduced stability to high temperature, low pH, and biliary salts; and in an increased efficacy of cell entry. In conclusion, the adaptation to cellular shutoff resulted in the selection of large-plaque-producing virus populations.
Importance: HAV has a naturally deoptimized codon usage with respect to that of its cell host and is unable to shut down the cellular translation. This fact contributes to the low replication rate of the virus, in addition to other factors such as the highly inefficient internal ribosome entry site (IRES), and explains the outstanding physical stability of this pathogen in the environment mediated by a folding-dependent highly cohesive capsid. Adaptation to artificially induced cellular transcription shutoff resulted in a redeoptimization of its capsid codon usage, instead of an optimization. These genomic changes are related to an overall change of capsid folding, which in turn induces changes in the cell entry process. Remarkably, the adaptation to cellular shutoff allowed the virus to significantly increase its RNA uncoating efficiency, resulting in the selection of large-plaque-producing populations. However, these populations produced much-debilitated virions.
Figures

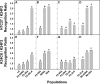
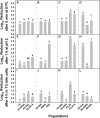

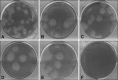
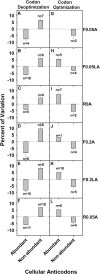
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

