Dysgranular retrosplenial cortex lesions in rats disrupt cross-modal object recognition
- PMID: 24554671
- PMCID: PMC3929849
- DOI: 10.1101/lm.032516.113
Dysgranular retrosplenial cortex lesions in rats disrupt cross-modal object recognition
Abstract
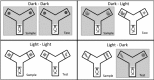
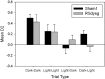
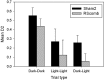
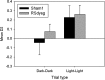
The retrosplenial cortex supports navigation, with one role thought to be the integration of different spatial cue types. This hypothesis was extended by examining the integration of nonspatial cues. Rats with lesions in either the dysgranular subregion of retrosplenial cortex (area 30) or lesions in both the granular and dysgranular subregions (areas 29 and 30) were tested on cross-modal object recognition (Experiment 1). In these tests, rats used different sensory modalities when exploring and subsequently recognizing the same test objects. The objects were first presented either in the dark, i.e., giving tactile and olfactory cues, or in the light behind a clear Perspex barrier, i.e., giving visual cues. Animals were then tested with either constant combinations of sample and test conditions (light to light, dark to dark), or changed "cross-modal" combinations (light to dark, dark to light). In Experiment 2, visual object recognition was tested without Perspex barriers, but using objects that could not be distinguished in the dark. The dysgranular retrosplenial cortex lesions selectively impaired cross-modal recognition when cue conditions switched from dark to light between initial sampling and subsequent object recognition, but no impairment was seen when the cue conditions remained constant, whether dark or light. The combined (areas 29 and 30) lesioned rats also failed the dark to light cross-modal problem but this impairment was less selective. The present findings suggest a role for the dysgranular retrosplenial cortex in mediating the integration of information across multiple cue types, a role that potentially applies to both spatial and nonspatial domains.
Figures



Similar articles
-
The rat retrosplenial cortex is required when visual cues are used flexibly to determine location.Behav Brain Res. 2014 Apr 15;263(100):98-107. doi: 10.1016/j.bbr.2014.01.028. Epub 2014 Jan 29. Behav Brain Res. 2014. PMID: 24486256 Free PMC article.
-
Granular and dysgranular retrosplenial cortices provide qualitatively different contributions to spatial working memory: evidence from immediate-early gene imaging in rats.Eur J Neurosci. 2009 Sep;30(5):877-88. doi: 10.1111/j.1460-9568.2009.06881.x. Epub 2009 Aug 27. Eur J Neurosci. 2009. PMID: 19712100
-
Effects of selective granular retrosplenial cortex lesions on spatial working memory in rats.Behav Brain Res. 2010 Apr 2;208(2):566-75. doi: 10.1016/j.bbr.2010.01.001. Epub 2010 Jan 12. Behav Brain Res. 2010. PMID: 20074589
-
The separate and combined properties of the granular (area 29) and dysgranular (area 30) retrosplenial cortex.Neurobiol Learn Mem. 2021 Nov;185:107516. doi: 10.1016/j.nlm.2021.107516. Epub 2021 Sep 3. Neurobiol Learn Mem. 2021. PMID: 34481970 Review.
-
Retrosplenial cortex and its role in cue-specific learning and memory.Neurosci Biobehav Rev. 2019 Dec;107:713-728. doi: 10.1016/j.neubiorev.2019.04.016. Epub 2019 May 2. Neurosci Biobehav Rev. 2019. PMID: 31055014 Free PMC article. Review.
Cited by
-
Differentiated somatic gene expression is triggered in the dorsal hippocampus and the anterior retrosplenial cortex by hippocampal synaptic plasticity prompted by spatial content learning.Brain Struct Funct. 2024 Apr;229(3):639-655. doi: 10.1007/s00429-023-02694-z. Epub 2023 Sep 10. Brain Struct Funct. 2024. PMID: 37690045 Free PMC article.
-
Unique roles for the anterior and posterior retrosplenial cortices in encoding and retrieval of memory for context.Cereb Cortex. 2022 Aug 22;32(17):3602-3610. doi: 10.1093/cercor/bhab436. Cereb Cortex. 2022. PMID: 35029643 Free PMC article.
-
The rat retrosplenial cortex as a link for frontal functions: A lesion analysis.Behav Brain Res. 2017 Sep 29;335:88-102. doi: 10.1016/j.bbr.2017.08.010. Epub 2017 Aug 8. Behav Brain Res. 2017. PMID: 28797600 Free PMC article.
-
Unexpected Terrain Induced Changes in Cortical Activity in Bipedal-Walking Rats.Biology (Basel). 2021 Dec 27;11(1):36. doi: 10.3390/biology11010036. Biology (Basel). 2021. PMID: 35053035 Free PMC article.
-
Neuronal circuitry for recognition memory of object and place in rodent models.Neurosci Biobehav Rev. 2022 Oct;141:104855. doi: 10.1016/j.neubiorev.2022.104855. Epub 2022 Sep 8. Neurosci Biobehav Rev. 2022. PMID: 36089106 Free PMC article. Review.
References
-
- Aggleton JP 2010. Understanding retrosplenial amnesia: Insights from animal studies. Neuropsychologia 48: 2328–2338 - PubMed
-
- Banati RB, Goerres GW, Tjoa C, Aggleton JP, Grasby P 2000. The functional anatomy of visual-tactile integration in man: A study using positron emission tomography. Neuropsychologia 38: 115–124 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
