Slit diaphragm protein Neph1 and its signaling: a novel therapeutic target for protection of podocytes against glomerular injury
- PMID: 24554715
- PMCID: PMC3975002
- DOI: 10.1074/jbc.M113.505743
Slit diaphragm protein Neph1 and its signaling: a novel therapeutic target for protection of podocytes against glomerular injury
Abstract
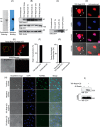
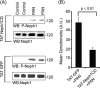
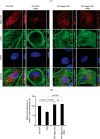
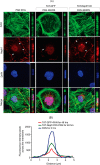
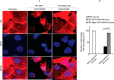
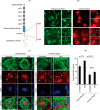
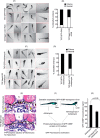
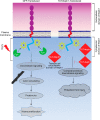
Podocytes are specialized epithelial cells that are critical components of the glomerular filtration barrier, and their dysfunction leads to proteinuria and renal failure. Therefore, preserving podocyte function is therapeutically significant. In this study, we identified Neph1 signaling as a therapeutic target that upon inhibition prevented podocyte damage from a glomerular injury-inducing agent puromycin aminonucleoside (PAN). To specifically inhibit Neph1 signaling, we used a protein transduction approach, where the cytoplasmic domain of Neph1 (Neph1CD) tagged with a protein transduction domain trans-activator of transcription was transduced in cultured podocytes prior to treatment with PAN. The PAN-induced Neph1 phosphorylation was significantly reduced in Neph1CD-transduced cells; in addition, these cells were resistant to PAN-induced cytoskeletal damage. The biochemical analysis using subfractionation studies showed that unlike control cells Neph1 was retained in the lipid raft fractions in the transduced cells following treatment with PAN, indicating that transduction of Neph1CD in podocytes prevented PAN-induced mislocalization of Neph1. In accordance, the immunofluorescence analysis further suggested that Neph1CD-transduced cells had increased ability to retain endogenous Neph1 at the membrane in response to PAN-induced injury. Similar results were obtained when angiotensin was used as an injury-inducing agent. Consistent with these observations, maintaining high levels of Neph1 at the membrane using a podocyte cell line overexpressing chimeric Neph1 increased the ability of podocytes to resist PAN-induced injury and PAN-induced albumin leakage. Using a zebrafish in vivo PAN and adriamycin injury models, we further demonstrated the ability of transduced Neph1CD to preserve glomerular function. Collectively, these results support the conclusion that inhibiting Neph1 signaling is therapeutically significant in preventing podocyte damage from glomerular injury.
Keywords: Actin; Cell Signaling; Kidney; Neph1; Phosphorylation; Podocytes; Renal Injury.
Figures


References
-
- Haraldsson B., Nyström J., Deen W. M. (2008) Properties of the glomerular barrier and mechanisms of proteinuria. Physiol. Rev. 88, 451–487 - PubMed
-
- Pavenstädt H., Kriz W., Kretzler M. (2003) Cell biology of the glomerular podocyte. Physiol. Rev. 83, 253–307 - PubMed
-
- Tryggvason K., Patrakka J., Wartiovaara J. (2006) Hereditary proteinuria syndromes and mechanisms of proteinuria. N. Engl. J. Med. 354, 1387–1401 - PubMed
-
- Tryggvason K., Pikkarainen T., Patrakka J. (2006) Nck links nephrin to actin in kidney podocytes. Cell 125, 221–224 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

