Lens crystallin modifications and cataract in transgenic mice overexpressing acylpeptide hydrolase
- PMID: 24554718
- PMCID: PMC3979366
- DOI: 10.1074/jbc.M113.510677
Lens crystallin modifications and cataract in transgenic mice overexpressing acylpeptide hydrolase
Abstract
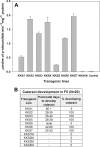
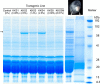
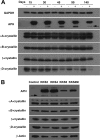
The accumulation of crystallin fragments in vivo and their subsequent interaction with crystallins are responsible, in part, for protein aggregation in cataracts. Transgenic mice overexpressing acylpeptide hydrolase (APH) specifically in the lens were prepared to test the role of protease in the generation and accumulation of peptides. Cataract development was seen at various postnatal days in the majority of mice expressing active APH (wt-APH). Cataract onset and severity of the cataracts correlated with the APH protein levels. Lens opacity occurred when APH protein levels were >2.6% of the total lens protein and the specific activity, assayed using Ac-Ala-p-nitroanilide substrate, was >1 unit. Transgenic mice carrying inactive APH (mt-APH) did not develop cataract. Cataract development also correlated with N-terminal cleavage of the APH to generate a 57-kDa protein, along with an increased accumulation of low molecular weight (LMW) peptides, similar to those found in aging human and cataract lenses. Nontransgenic mouse lens proteins incubated with purified wt-APH in vitro resulted in a >20% increase in LMW peptides. Crystallin modifications and cleavage were quite dramatic in transgenic mouse lenses with mature cataract. Affected lenses showed capsule rupture at the posterior pole, with expulsion of the lens nucleus and degenerating fiber cells. Our study suggests that the cleaved APH fragment might exert catalytic activity against crystallins, resulting in the accumulation of distinct LMW peptides that promote protein aggregation in lenses expressing wt-APH. The APH transgenic model we developed will enable in vivo testing of the roles of crystallin fragments in protein aggregation.
Keywords: Acylpeptide Hydrolase; Cataract; Crystallins; Lens; Peptides; Protease; Protein Degradation; Transgenic Mice.
Figures




Similar articles
-
Significance of interactions of low molecular weight crystallin fragments in lens aging and cataract formation.J Biol Chem. 2008 Mar 28;283(13):8477-85. doi: 10.1074/jbc.M705876200. Epub 2008 Jan 28. J Biol Chem. 2008. PMID: 18227073 Free PMC article.
-
Profiling of lens protease involved in generation of αA-66-80 crystallin peptide using an internally quenched protease substrate.Exp Eye Res. 2013 Apr;109:51-9. doi: 10.1016/j.exer.2013.01.016. Epub 2013 Feb 11. Exp Eye Res. 2013. PMID: 23410823 Free PMC article.
-
Measurement of absolute abundance of crystallins in human and αA N101D transgenic mouse lenses using 15N-labeled crystallin standards.Exp Eye Res. 2024 Nov;248:110115. doi: 10.1016/j.exer.2024.110115. Epub 2024 Oct 3. Exp Eye Res. 2024. PMID: 39368693
-
Isomerization of aspartyl residues in crystallins and its influence upon cataract.Biochim Biophys Acta. 2016 Jan;1860(1 Pt B):183-91. doi: 10.1016/j.bbagen.2015.08.001. Epub 2015 Aug 12. Biochim Biophys Acta. 2016. PMID: 26275494 Review.
-
Lens aging: effects of crystallins.Biochim Biophys Acta. 2009 Oct;1790(10):1095-108. doi: 10.1016/j.bbagen.2009.05.008. Epub 2009 May 20. Biochim Biophys Acta. 2009. PMID: 19463898 Free PMC article. Review.
Cited by
-
Expression, purification, crystallization and preliminary X-ray diffraction analysis of acylpeptide hydrolase from Deinococcus radiodurans.Acta Crystallogr F Struct Biol Commun. 2014 Sep;70(Pt 9):1292-5. doi: 10.1107/S2053230X14017944. Epub 2014 Aug 27. Acta Crystallogr F Struct Biol Commun. 2014. PMID: 25195912 Free PMC article.
-
In Vivo Sub-chronic Treatment with Dichlorvos in Young Rats Promotes Synaptic Plasticity and Learning by a Mechanism that Involves Acylpeptide Hydrolase Instead of Acetylcholinesterase Inhibition. Correlation with Endogenous β-Amyloid Levels.Front Pharmacol. 2017 Jul 25;8:483. doi: 10.3389/fphar.2017.00483. eCollection 2017. Front Pharmacol. 2017. PMID: 28790916 Free PMC article.
-
Imbalances in the eye lens proteome are linked to cataract formation.Nat Struct Mol Biol. 2021 Feb;28(2):143-151. doi: 10.1038/s41594-020-00543-9. Epub 2021 Jan 11. Nat Struct Mol Biol. 2021. PMID: 33432246
-
Oxidation of active cysteines mediates protein aggregation of S10R, the cataract-associated mutant of mouse GammaB-crystallin.Proteins. 2022 Nov;90(11):1987-2000. doi: 10.1002/prot.26391. Epub 2022 Jul 7. Proteins. 2022. PMID: 35726360 Free PMC article.
-
Macrophage recruitment in immune-privileged lens during capsule repair, necrotic fiber removal, and fibrosis.iScience. 2021 May 12;24(6):102533. doi: 10.1016/j.isci.2021.102533. eCollection 2021 Jun 25. iScience. 2021. PMID: 34142044 Free PMC article.
References
-
- Hoehenwarter W., Klose J., Jungblut P. R. (2006) Eye lens proteomics. Amino Acids 30, 369–389 - PubMed
-
- Murakami K., Jahngen J. H., Lin S. W., Davies K. J., Taylor A. (1990) Lens proteasome shows enhanced rates of degradation of hydroxyl radical modified α-crystallin. Free Radic. Biol. Med. 8, 217–222 - PubMed
-
- Davies K. J. (1990) Protein oxidation and proteolytic degradation. General aspects and relationship to cataract formation. Adv. Exp. Med. Biol. 264, 503–511 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

