Calcium co-regulates oxidative metabolism and ATP synthase-dependent respiration in pancreatic beta cells
- PMID: 24554722
- PMCID: PMC3979381
- DOI: 10.1074/jbc.M113.513184
Calcium co-regulates oxidative metabolism and ATP synthase-dependent respiration in pancreatic beta cells
Abstract
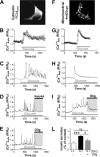
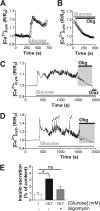
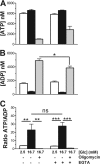
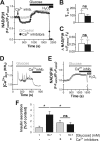
Mitochondrial energy metabolism is essential for glucose-induced calcium signaling and, therefore, insulin granule exocytosis in pancreatic beta cells. Calcium signals are sensed by mitochondria acting in concert with mitochondrial substrates for the full activation of the organelle. Here we have studied glucose-induced calcium signaling and energy metabolism in INS-1E insulinoma cells and human islet beta cells. In insulin secreting cells a surprisingly large fraction of total respiration under resting conditions is ATP synthase-independent. We observe that ATP synthase-dependent respiration is markedly increased after glucose stimulation. Glucose also causes a very rapid elevation of oxidative metabolism as was followed by NAD(P)H autofluorescence. However, neither the rate of the glucose-induced increase nor the new steady-state NAD(P)H levels are significantly affected by calcium. Our findings challenge the current view, which has focused mainly on calcium-sensitive dehydrogenases as the target for the activation of mitochondrial energy metabolism. We propose a model of tight calcium-dependent regulation of oxidative metabolism and ATP synthase-dependent respiration in beta cell mitochondria. Coordinated activation of matrix dehydrogenases and respiratory chain activity by calcium allows the respiratory rate to change severalfold with only small or no alterations of the NAD(P)H/NAD(P)(+) ratio.
Keywords: Calcium; Energy Metabolism; Insulin; Islet; Mitochondria; NAD.
Figures




References
-
- Balaban R. S., Kantor H. L., Katz L. A., Briggs R. W. (1986) Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science 232, 1121–1123 - PubMed
-
- Sweet I. R., Cook D. L., DeJulio E., Wallen A. R., Khalil G., Callis J., Reems J. (2004) Regulation of ATP/ADP in pancreatic islets. Diabetes 53, 401–409 - PubMed
-
- Wiederkehr A., Szanda G., Akhmedov D., Mataki C., Heizmann C. W., Schoonjans K., Pozzan T., Spät A., Wollheim C. B. (2011) Mitochondrial matrix calcium is an activating signal for hormone secretion. Cell Metab. 13, 601–611 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

