Evidence for proteomic and metabolic adaptations associated with alterations of seed yield and quality in sulfur-limited Brassica napus L
- PMID: 24554741
- PMCID: PMC4014277
- DOI: 10.1074/mcp.M113.034215
Evidence for proteomic and metabolic adaptations associated with alterations of seed yield and quality in sulfur-limited Brassica napus L
Abstract
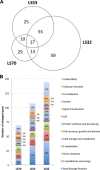
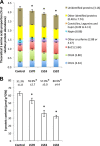
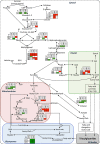
In Brassica napus, seed yield and quality are related to sulfate availability, but the seed metabolic changes in response to sulfate limitation remain largely unknown. To address this question, proteomics and biochemical studies were carried out on mature seeds obtained from plants grown under low sulfate applied at the bolting (LS32), early flowering (LS53), or start of pod filling (LS70) stage. The protein quality of all low-sulfate seeds was reduced and associated with a reduction of S-rich seed storage protein accumulation (as Cruciferin Cru4) and an increase of S-poor seed storage protein (as Cruciferin BnC1). This compensation allowed the protein content to be maintained in LS70 and LS53 seeds but was not sufficient to maintain the protein content in LS32 seeds. The lipid content and quality of LS53 and LS32 seeds were also affected, and these effects were primarily associated with a reduction of C18-derivative accumulation. Proteomics changes related to lipid storage, carbohydrate metabolism, and energy (reduction of caleosins, phosphoglycerate kinase, malate synthase, ATP-synthase β-subunit, and thiazole biosynthetic enzyme THI1 and accumulation of β-glucosidase and citrate synthase) provide insights into processes that may contribute to decreased oil content and altered lipid composition (in favor of long-chain fatty acids in LS53 and LS32 seeds). These data indicate that metabolic changes associated with S limitation responses affect seed storage protein composition and lipid quality. Proteins involved in plant stress response, such as dehydroascorbate reductase and Cu/Zn-superoxide dismutase, were also accumulated in LS53 and LS32 seeds, and this might be a consequence of reduced glutathione content under low S availability. LS32 treatment also resulted in (i) reduced germination vigor, as evidenced by lower germination indexes, (ii) reduced seed germination capacity, related to a lower seed viability, and (iii) a strong decrease of glyoxysomal malate synthase, which is essential for the use of fatty acids during seedling establishment.
Figures



References
-
- McGrath S. P., Zhao F.-J. (1996) Sulphur uptake, yield responses and the interactions between nitrogen and sulphur in winter oilseed rape (Brassica napus). J. Agr. Sci. 126, 53–62
-
- Zhao F.-J., Bilsborrow P. E., Evans E. J., McGrath S. P. (1997) Nitrogen to sulphur ratio in rapeseed and in rapeseed protein and its use in diagnosing sulphur deficiency. J. Plant Nutr. 20, 549–558
-
- Scherer H. W. (2001) Sulphur in crop production—invited paper. Eur. J. Agron. 14, 81–111
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

