In vivo and in vitro analyses of amygdalar function reveal a role for copper
- PMID: 24554785
- PMCID: PMC4044343
- DOI: 10.1152/jn.00631.2013
In vivo and in vitro analyses of amygdalar function reveal a role for copper
Abstract
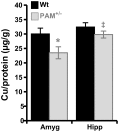
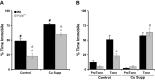
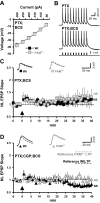
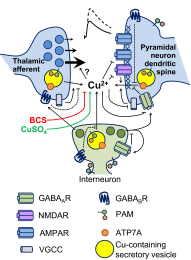
Mice with a single copy of the peptide amidating monooxygenase (Pam) gene (PAM(+/-)) are impaired in contextual and cued fear conditioning. These abnormalities coincide with deficient long-term potentiation (LTP) at excitatory thalamic afferent synapses onto pyramidal neurons in the lateral amygdala. Slice recordings from PAM(+/-) mice identified an increase in GABAergic tone (Gaier ED, Rodriguiz RM, Ma XM, Sivaramakrishnan S, Bousquet-Moore D, Wetsel WC, Eipper BA, Mains RE. J Neurosci 30: 13656-13669, 2010). Biochemical data indicate a tissue-specific deficit in Cu content in the amygdala; amygdalar expression of Atox-1 and Atp7a, essential for transport of Cu into the secretory pathway, is reduced in PAM(+/-) mice. When PAM(+/-) mice were fed a diet supplemented with Cu, the impairments in fear conditioning were reversed, and LTP was normalized in amygdala slice recordings. A role for endogenous Cu in amygdalar LTP was established by the inhibitory effect of a brief incubation of wild-type slices with bathocuproine disulfonate, a highly selective, cell-impermeant Cu chelator. Interestingly, bath-applied CuSO₄ had no effect on excitatory currents but reversibly potentiated the disynaptic inhibitory current. Bath-applied CuSO₄ was sufficient to potentiate wild-type amygdala afferent synapses. The ability of dietary Cu to affect signaling in pathways that govern fear-based behaviors supports an essential physiological role for Cu in amygdalar function at both the synaptic and behavioral levels. This work is relevant to neurological and psychiatric disorders in which disturbed Cu homeostasis could contribute to altered synaptic transmission, including Wilson's, Menkes, Alzheimer's, and prion-related diseases.
Keywords: GABA; fear; learning and memory; peptidylglycine alpha-amidating monooxygenase; synaptic plasticity.
Copyright © 2014 the American Physiological Society.
Figures


Similar articles
-
Pam heterozygous mice reveal essential role for Cu in amygdalar behavioral and synaptic function.Ann N Y Acad Sci. 2014 May;1314(1):15-23. doi: 10.1111/nyas.12378. Epub 2014 Mar 4. Ann N Y Acad Sci. 2014. PMID: 24593825 Free PMC article.
-
Peptidylglycine α-amidating monooxygenase heterozygosity alters brain copper handling with region specificity.J Neurochem. 2013 Dec;127(5):605-19. doi: 10.1111/jnc.12438. Epub 2013 Oct 13. J Neurochem. 2013. PMID: 24032518 Free PMC article.
-
Haploinsufficiency in peptidylglycine alpha-amidating monooxygenase leads to altered synaptic transmission in the amygdala and impaired emotional responses.J Neurosci. 2010 Oct 13;30(41):13656-69. doi: 10.1523/JNEUROSCI.2200-10.2010. J Neurosci. 2010. PMID: 20943906 Free PMC article.
-
Synaptic transmission and plasticity in the amygdala. An emerging physiology of fear conditioning circuits.Mol Neurobiol. 1996 Aug;13(1):1-22. doi: 10.1007/BF02740749. Mol Neurobiol. 1996. PMID: 8892333 Review.
-
Synaptic mechanisms of associative memory in the amygdala.Neuron. 2005 Sep 15;47(6):783-6. doi: 10.1016/j.neuron.2005.08.009. Neuron. 2005. PMID: 16157273 Review.
Cited by
-
Copper-uptake is critical for the down regulation of synapsin and dynamin induced by neocuproine: modulation of synaptic activity in hippocampal neurons.Front Aging Neurosci. 2014 Dec 3;6:319. doi: 10.3389/fnagi.2014.00319. eCollection 2014. Front Aging Neurosci. 2014. PMID: 25520655 Free PMC article.
-
Identifying roles for peptidergic signaling in mice.Proc Natl Acad Sci U S A. 2019 Oct 1;116(40):20169-20179. doi: 10.1073/pnas.1910495116. Epub 2019 Aug 27. Proc Natl Acad Sci U S A. 2019. PMID: 31455734 Free PMC article.
-
Stabilization of Aliphatic Phosphines by Auxiliary Phosphine Sulfides Offers Zeptomolar Affinity and Unprecedented Selectivity for Probing Biological CuI.Angew Chem Int Ed Engl. 2018 Jul 26;57(31):9711-9715. doi: 10.1002/anie.201804072. Epub 2018 Jul 12. Angew Chem Int Ed Engl. 2018. PMID: 29885022 Free PMC article.
-
Analytical Methods for Imaging Metals in Biology: From Transition Metal Metabolism to Transition Metal Signaling.Anal Chem. 2017 Jan 3;89(1):22-41. doi: 10.1021/acs.analchem.6b04631. Epub 2016 Dec 15. Anal Chem. 2017. PMID: 27976855 Free PMC article. Review. No abstract available.
-
Memory and Learning Dysfunction Following Copper Toxicity: Biochemical and Immunohistochemical Basis.Mol Neurobiol. 2018 May;55(5):3800-3811. doi: 10.1007/s12035-017-0619-y. Epub 2017 May 23. Mol Neurobiol. 2018. PMID: 28536976
References
-
- Akatsu H, Hori A, Yamamoto T, Yoshida M, Mimuro M, Hashizume Y, Tooyama I, Yezdimer EM. Transition metal abnormalities in progressive dementias. Biometals 25: 337–350, 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

