Diethylentriaminepenta acetic acid glucose conjugates as a cell permeable iron chelator
- PMID: 24554907
- PMCID: PMC3917162
- DOI: 10.4103/0976-500X.124416
Diethylentriaminepenta acetic acid glucose conjugates as a cell permeable iron chelator
Abstract
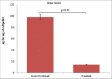
Objective: To find out whether DTPA-DG complex can enhance clearance of intracellular free iron.
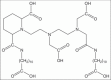
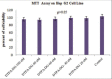
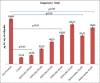
Materials and methods: Diethylenetriaminepentaacetic acid-D-deoxy-glucosamine (DTPA-DG) was synthesized and examined for its activity as a cell-permeable iron chelator in human hepatocellular carcinoma (HEPG2) cell line exposed to high concentration of iron sulfate and compared with deferoxamine (DFO), a prototype iron chelator. The effect of DTPA-DG on cell viability was monitored using the 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide MTT assay as well.
Results: There was a significant increase of iron level after iron overload induction in HEPG2 cell culture. DTPA-DG presented a remarkable capacity to iron burden reducing with estimated 50% inhibitory concentration value of 65.77 nM. In fact, glycosyl moiety was gained access of DTPA to intracellular iron deposits through glucose transporter systems.
Conclusion: DTPA-DG, more potent than DFO to sequester deposits of free iron with no profound toxic effect. The results suggest the potential of DTPA-DG in chelating iron and permitting its excretion from primary organ storage.
Keywords: Cell-permeable; glycosyl moiety; human hepatocellular carcinoma; iron chelation; iron overload.
Conflict of interest statement
Figures




Similar articles
-
Polymeric Nanoparticles Enhance the Ability of Deferoxamine To Deplete Hepatic and Systemic Iron.Nano Lett. 2018 Sep 12;18(9):5782-5790. doi: 10.1021/acs.nanolett.8b02428. Epub 2018 Aug 9. Nano Lett. 2018. PMID: 30085676
-
Cytoprotection and iron mobilization in rat hepatocyte cultures by a new synthetic dihydroxamate chelator.Toxicol Lett. 1999 Oct 29;110(1-2):19-27. doi: 10.1016/s0378-4274(99)00135-6. Toxicol Lett. 1999. PMID: 10593591
-
The effect of the iron(III) chelator, desferrioxamine, on iron and transferrin uptake by the human malignant melanoma cell.Cancer Res. 1994 Feb 1;54(3):685-9. Cancer Res. 1994. PMID: 8306330
-
Objectives and mechanism of iron chelation therapy.Ann N Y Acad Sci. 2005;1054:124-35. doi: 10.1196/annals.1345.015. Ann N Y Acad Sci. 2005. PMID: 16339658 Review.
-
Chelation therapy in beta-thalassemia: an optimistic update.Semin Hematol. 2001 Oct;38(4):360-6. doi: 10.1016/s0037-1963(01)90030-7. Semin Hematol. 2001. PMID: 11605171 Review.
Cited by
-
Morphine-Induced Modulation of Endolysosomal Iron Mediates Upregulation of Ferritin Heavy Chain in Cortical Neurons.eNeuro. 2019 Jul 31;6(4):ENEURO.0237-19.2019. doi: 10.1523/ENEURO.0237-19.2019. Print 2019 Jul/Aug. eNeuro. 2019. PMID: 31300544 Free PMC article.
References
-
- Felitti VJ. Hemochromatosis: A common, rarely diagnosed disease. Perm J. 1999;3:10–22.
-
- Merryweather-Clarke AT, Pointon JJ, Jouanolle AM, Rochette J, Robson KJ. Geography of HFE C282Y and H63D mutations. Genet Test. 2000;4:183–98. - PubMed
-
- Ryan E, O’keane C, Crowe J. Hemochromatosis in Ireland and HFE. Blood Cells Mol Dis. 1998;24:428–32. - PubMed
-
- Brissot P. Diagnosis and current treatments for primary iron overload. Am J Hematol. 2007;82:1140–1. - PubMed
-
- Payne KA, Rofail D, Baladi JF, Viala M, Abetz L, Desrosiers MP, et al. Iron chelation therapy: Clinical effectiveness, economic burden and quality of life in patients with iron overload. Adv Ther. 2008;25:725–42. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
