Evaluation of treatment of invasive fungal infections
- PMID: 24554910
- PMCID: PMC3917166
- DOI: 10.4103/0976-500X.124423
Evaluation of treatment of invasive fungal infections
Abstract
Objective: To identify the risk factors associated with invasive fungal infections (IFI) in immunocompromised patients (IP), and monitor antifungal therapy appropriateness and costs.
Materials and methods: The 1-year observational retrospective study was performed on 101 IP, who received antifungal intravenous therapy with fluconazole (F), liposomal amphotericin-B (A), caspofungin (C), itraconazole (I) for ≥4 days. Patient therapy was divided into three groups: Prophylactic, empirical, and target. Immunosuppressive therapy (IT), total parenteral nutrition (TPN), dialysis, central line, steroid therapy, stent use, neutropenia, and mechanical ventilation were evaluated. Variables were therapy duration, defined daily dose (DDD) consumption, DDD average cost.
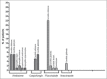
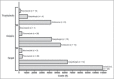
Results: Main risk factors were central line (65.3%), TPN (56.4%), dialysis (46.5%), IT (42.6%), mechanical ventilation (32.7%), neutropenia (24.8%), steroid therapy (23.8%), and stent use (14.9%). Average duration of prophylaxis was 7 days; F (61%), A (26%), and C (13%) were used. Average duration of empirical therapy was 8 days; F (52.9%), A (26.5%), C (8.8%), I (2.9%), and in association A + C, A + F, C + F (8.9%) were used. Average duration of target therapy was 9 days; F (40.4%), A (23.1%), C (15.4%), I (7.7%), and in association A + C, A + F, C + F (13.4%) were used. DDD consumption and DDD average-cost were: C 50 mg vial: 273 DDD, €381.1; C 70 mg vial: 33.6 DDD, €389.6; F 200 mg vial: 768 DDD, €11.8; F 100 mg vial: 89 DDD, €10.6; I 250 mg vials: 62.5 DDD, €68.8; and A 50 mg vial: 2200 DDD, €93.4; respectively.
Conclusions: Data showed an appropriate use of antifungals. Best alternative therapy (cheaper antifungal drug) was prescribed for most patients. The high cost of A and C was justified by IFI resolution.
Keywords: Costs; defined daily dose; invasive fungal infections; prescriptive appropriateness; risk factors.
Conflict of interest statement
Figures
Similar articles
-
Liposomal amphotericin B: a review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections.Drugs. 2009;69(3):361-92. doi: 10.2165/00003495-200969030-00010. Drugs. 2009. PMID: 19275278 Review.
-
EPICO 3.0. Empirical antifungal therapy in critically-ill hematology patients.Rev Iberoam Micol. 2016 Oct-Dec;33(4):206-215. doi: 10.1016/j.riam.2016.06.002. Epub 2016 Oct 15. Rev Iberoam Micol. 2016. PMID: 27751781
-
Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia.N Engl J Med. 2004 Sep 30;351(14):1391-402. doi: 10.1056/NEJMoa040446. N Engl J Med. 2004. PMID: 15459300 Clinical Trial.
-
Itraconazole oral solution for primary prophylaxis of fungal infections in patients with hematological malignancy and profound neutropenia: a randomized, double-blind, double-placebo, multicenter trial comparing itraconazole and amphotericin B.Antimicrob Agents Chemother. 2000 Jul;44(7):1887-93. doi: 10.1128/AAC.44.7.1887-1893.2000. Antimicrob Agents Chemother. 2000. PMID: 10858349 Free PMC article. Clinical Trial.
-
Patients at high risk of invasive fungal infections: when and how to treat.Drugs. 2008;68(14):1941-62. doi: 10.2165/00003495-200868140-00002. Drugs. 2008. PMID: 18778118 Review.
Cited by
-
Potentially inappropriate drug prescribing in elderly hospitalized patients: an analysis and comparison of explicit criteria.Int J Clin Pharm. 2016 Apr;38(2):462-8. doi: 10.1007/s11096-016-0284-7. Epub 2016 Mar 16. Int J Clin Pharm. 2016. PMID: 26984238
-
Efficacy of Posaconazole Prophylaxis for Fungal Disease in Hematology Patients Treated With Chemotherapy and Transplantation: An Open-Label, Prospective, Observational Study.Front Microbiol. 2020 Mar 19;11:349. doi: 10.3389/fmicb.2020.00349. eCollection 2020. Front Microbiol. 2020. PMID: 32265849 Free PMC article.
References
-
- Maertens J. Antifungal therapy, a challenge in the management of immunocompromised patients. Eur J Hosp Pharm Prac. 2007;13:16.
-
- Fortún J, Ruiz I, Martín-Dávila P, Cuenca-Estrella M. Fungal infection in solid organ recipients. Enferm Infecc Microbiol Clin. 2012;30(Suppl 2):49–56. - PubMed
-
- Paloušová D, Lengerová M, Volfová P, Bejdák P, Kocmanová I, Mayer J, et al. Invasive fungal infections in immunocompromised patients with focus on aspergillosis and its causative agents. Klin Mikrobiol Infekc Lek. 2012;18:96–101. - PubMed
-
- Quindós G. Candidiasis, aspergillosis and other invasive mycoses in recipients of solid organ transplants. Rev Iberoam Micol. 2011;28:110–9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials