Process-induced extracellular matrix alterations affect the mechanisms of soft tissue repair and regeneration
- PMID: 24555005
- PMCID: PMC3927753
- DOI: 10.1177/2041731413505305
Process-induced extracellular matrix alterations affect the mechanisms of soft tissue repair and regeneration
Abstract
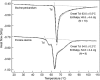
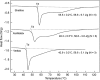
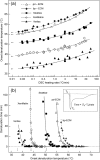
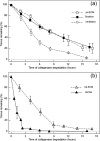
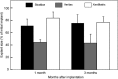
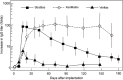
Extracellular matrices derived from animal tissues for human tissue repairs are processed by various methods of physical, chemical, or enzymatic decellularization, viral inactivation, and terminal sterilization. The mechanisms of action in tissue repair vary among bioscaffolds and are suggested to be associated with process-induced extracellular matrix modifications. We compared three non-cross-linked, commercially available extracellular matrix scaffolds (Strattice, Veritas, and XenMatrix), and correlated extracellular matrix alterations to in vivo biological responses upon implantation in non-human primates. Structural evaluation showed significant differences in retaining native tissue extracellular matrix histology and ultrastructural features among bioscaffolds. Tissue processing may cause both the condensation of collagen fibers and fragmentation or separation of collagen bundles. Calorimetric analysis showed significant differences in the stability of bioscaffolds. The intrinsic denaturation temperature was measured to be 51°C, 38°C, and 44°C for Strattice, Veritas, and XenMatrix, respectively, demonstrating more extracellular matrix modifications in the Veritas and XenMatrix scaffolds. Consequently, the susceptibility to collagenase degradation was increased in Veritas and XenMatrix when compared to their respective source tissues. Using a non-human primate model, three bioscaffolds were found to elicit different biological responses, have distinct mechanisms of action, and yield various outcomes of tissue repair. Strattice permitted cell repopulation and was remodeled over 6 months. Veritas was unstable at body temperature, resulting in rapid absorption with moderate inflammation. XenMatrix caused severe inflammation and sustained immune reactions. This study demonstrates that extracellular matrix alterations significantly affect biological responses in soft tissue repair and regeneration. The data offer useful insights into the rational design of extracellular matrix products and bioscaffolds of tissue engineering.
Keywords: Biological scaffold; bovine pericardium; decellularization; extracellular matrix; matrix remodeling; porcine dermis; soft tissue regeneration; tissue engineering.
Conflict of interest statement
Figures



Similar articles
-
A porcine-derived acellular dermal scaffold that supports soft tissue regeneration: removal of terminal galactose-alpha-(1,3)-galactose and retention of matrix structure.Tissue Eng Part A. 2009 Jul;15(7):1807-19. doi: 10.1089/ten.tea.2008.0384. Tissue Eng Part A. 2009. PMID: 19196142
-
Effect of repetitive loading on the mechanical properties of biological scaffold materials.J Am Coll Surg. 2012 Aug;215(2):216-28. doi: 10.1016/j.jamcollsurg.2012.03.006. Epub 2012 Apr 21. J Am Coll Surg. 2012. PMID: 22521670
-
Comparison of Antibiotic-Coated versus Uncoated Porcine Dermal Matrix.Plast Reconstr Surg. 2016 Nov;138(5):844e-855e. doi: 10.1097/PRS.0000000000002688. Plast Reconstr Surg. 2016. PMID: 27782996
-
Xenogeneic extracellular matrix as a scaffold for tissue reconstruction.Transpl Immunol. 2004 Apr;12(3-4):367-77. doi: 10.1016/j.trim.2003.12.016. Transpl Immunol. 2004. PMID: 15157928 Review.
-
Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance.Methods. 2015 Aug;84:25-34. doi: 10.1016/j.ymeth.2015.03.005. Epub 2015 Mar 16. Methods. 2015. PMID: 25791470 Review.
Cited by
-
The Usage of Mesh and Relevant Prognosis in Implant Breast Reconstruction Surgery: A Meta-analysis.Aesthetic Plast Surg. 2024 Sep;48(17):3386-3399. doi: 10.1007/s00266-024-03879-5. Epub 2024 Mar 4. Aesthetic Plast Surg. 2024. PMID: 38438762
-
Laparoscopic repair of type III/IV giant para-oesophageal herniae with biological prosthesis: a single centre experience.Hernia. 2019 Apr;23(2):387-396. doi: 10.1007/s10029-019-01888-x. Epub 2019 Jan 19. Hernia. 2019. PMID: 30661178
-
Comparison of mechanical properties and host tissue response to OviTex™ and Strattice™ surgical meshes.Hernia. 2023 Aug;27(4):987-997. doi: 10.1007/s10029-023-02769-0. Epub 2023 Apr 8. Hernia. 2023. PMID: 37031315 Free PMC article.
-
Glycerolized Reticular Dermis as a New Human Acellular Dermal Matrix: An Exploratory Study.PLoS One. 2016 Feb 26;11(2):e0149124. doi: 10.1371/journal.pone.0149124. eCollection 2016. PLoS One. 2016. PMID: 26918526 Free PMC article.
-
Evaluation of Supercritical CO2-Assisted Protocols in a Model of Ovine Aortic Root Decellularization.Molecules. 2020 Aug 27;25(17):3923. doi: 10.3390/molecules25173923. Molecules. 2020. PMID: 32867356 Free PMC article.
References
-
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 2004; 4: 617–629 - PubMed
-
- Rieder E, Kasimir MT, Silberhumer G, et al. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiov Sur 2004; 127: 399–405 - PubMed
-
- Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biologic scaffold material: structure and function. Acta Biomater 2009; 5: 1–13 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

