Porcine cholecyst-derived scaffold promotes full-thickness wound healing in rabbit
- PMID: 24555014
- PMCID: PMC3927752
- DOI: 10.1177/2041731413518060
Porcine cholecyst-derived scaffold promotes full-thickness wound healing in rabbit
Abstract
Graft-assisted healing is an important strategy for treating full-thickness skin wounds. This study evaluated the properties of porcine cholecyst-derived scaffold and its use for treating full-thickness skin wound in rabbit. The physical properties of cholecyst-derived scaffold were congenial for skin-graft application. Compared to a commercially available skin-graft substitute made of porcine small intestinal submucosa, the cholecyst-derived scaffold was rich in natural biomolecules like elastin and glycosaminoglycans. When used as a xenograft, it promoted healing with excess cell proliferation at early phases and acceptable collagen deposition in the later remodelling phases.
Keywords: Wound healing; cholecyst-derived scaffold; collagen; extracellular matrix; graft-assisted healing; skin graft.
Conflict of interest statement
Figures

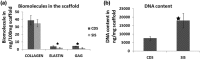










References
-
- Cronin H, Goldstein G. Biologic skin substitutes and their applications in dermatology. Dermatol Surg 2013; 39: 30–34 - PubMed
-
- Pereira C, Gold W, Herndon D. Review paper: burn coverage technologies: current concepts and future directions. J Biomater Appl 2007; 22: 101–121 - PubMed
-
- Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci 2008; 121: 255–264 - PubMed
-
- Reing JE, Zhang L, Myers-Irvin J, et al. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Pt A 2009; 15: 605–614 - PubMed
-
- Kleinman HK, Philp D, Hoffman MP. Role of the extracellular matrix in morphogenesis. Curr Opin Biotech 2003; 14: 526–532 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

