Contesting the dogma of an age-related heat shock response impairment: implications for cardiac-specific age-related disorders
- PMID: 24556212
- PMCID: PMC4065144
- DOI: 10.1093/hmg/ddu073
Contesting the dogma of an age-related heat shock response impairment: implications for cardiac-specific age-related disorders
Abstract
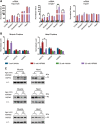
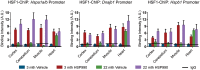
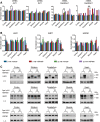
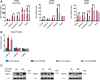
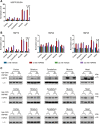
Ageing is associated with the reduced performance of physiological processes and has been proposed as a major risk factor for disease. An age-related decline in stress response pathways has been widely documented in lower organisms. In particular, the heat shock response (HSR) becomes severely compromised with age in Caenorhabditis elegans. However, a comprehensive analysis of the consequences of ageing on the HSR in higher organisms has not been documented. We used both HS and inhibition of HSP90 to induce the HSR in wild-type mice at 3 and 22 months of age to investigate the extent to which different brain regions, and peripheral tissues can sustain HSF1 activity and HS protein (HSP) expression with age. Using chromatin immunoprecipitation, quantitative reverse transcription polymerase chain reaction, western blotting and enzyme linked immunosorbent assay (ELISA), we were unable to detect a difference in the level or kinetics of HSP expression between young and old mice in all brain regions. In contrast, we did observe an age-related reduction in chaperone levels and HSR-related proteins in the heart. This could result in a decrease in the protein folding capacity of old hearts with implications for age-related cardiac disorders.
© The Author 2014. Published by Oxford University Press.
Figures




Similar articles
-
Heat shock factor 2 is required for maintaining proteostasis against febrile-range thermal stress and polyglutamine aggregation.Mol Biol Cell. 2011 Oct;22(19):3571-83. doi: 10.1091/mbc.E11-04-0330. Epub 2011 Aug 3. Mol Biol Cell. 2011. PMID: 21813737 Free PMC article.
-
The heat shock response, determined by QuantiGene multiplex, is impaired in HD mouse models and not caused by HSF1 reduction.Sci Rep. 2021 Apr 27;11(1):9117. doi: 10.1038/s41598-021-88715-5. Sci Rep. 2021. PMID: 33907289 Free PMC article.
-
RhoA Activation Sensitizes Cells to Proteotoxic Stimuli by Abrogating the HSF1-Dependent Heat Shock Response.PLoS One. 2015 Jul 20;10(7):e0133553. doi: 10.1371/journal.pone.0133553. eCollection 2015. PLoS One. 2015. PMID: 26193369 Free PMC article.
-
Regulation of heat shock gene transcription in neuronal cells.Int J Hyperthermia. 2005 Aug;21(5):433-44. doi: 10.1080/02656730500165514. Int J Hyperthermia. 2005. PMID: 16048840 Review.
-
Small molecule activators of the heat shock response: chemical properties, molecular targets, and therapeutic promise.Chem Res Toxicol. 2012 Oct 15;25(10):2036-53. doi: 10.1021/tx300264x. Epub 2012 Jul 31. Chem Res Toxicol. 2012. PMID: 22799889 Free PMC article. Review.
Cited by
-
Heat-Shock Proteins in Neuroinflammation.Front Pharmacol. 2019 Aug 27;10:920. doi: 10.3389/fphar.2019.00920. eCollection 2019. Front Pharmacol. 2019. PMID: 31507418 Free PMC article. Review.
-
Molecular Chaperones and Proteolytic Machineries Regulate Protein Homeostasis In Aging Cells.Cells. 2020 May 24;9(5):1308. doi: 10.3390/cells9051308. Cells. 2020. PMID: 32456366 Free PMC article. Review.
-
Heat Shock Factor 1 Prevents Age-Related Hearing Loss by Decreasing Endoplasmic Reticulum Stress.Cells. 2021 Sep 17;10(9):2454. doi: 10.3390/cells10092454. Cells. 2021. PMID: 34572102 Free PMC article.
-
Translational reprogramming in response to accumulating stressors ensures critical threshold levels of Hsp90 for mammalian life.Nat Commun. 2022 Oct 21;13(1):6271. doi: 10.1038/s41467-022-33916-3. Nat Commun. 2022. PMID: 36270993 Free PMC article.
-
Equine skeletal muscle adaptations to exercise and training: evidence of differential regulation of autophagosomal and mitochondrial components.BMC Genomics. 2017 Aug 9;18(1):595. doi: 10.1186/s12864-017-4007-9. BMC Genomics. 2017. PMID: 28793853 Free PMC article.
References
-
- Kenyon C.J. The genetics of ageing. Nature. 2010;464:504–512. - PubMed
-
- Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. - PubMed
-
- Haynes C.M., Ron D. The mitochondrial UPR - protecting organelle protein homeostasis. J. Cell Sci. 2010;123:3849–3855. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

