Cellular and molecular interactions of phosphoinositides and peripheral proteins
- PMID: 24556335
- PMCID: PMC4484752
- DOI: 10.1016/j.chemphyslip.2014.02.002
Cellular and molecular interactions of phosphoinositides and peripheral proteins
Abstract
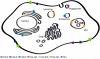
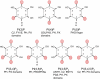
Anionic lipids act as signals for the recruitment of proteins containing cationic clusters to biological membranes. A family of anionic lipids known as the phosphoinositides (PIPs) are low in abundance, yet play a critical role in recruitment of peripheral proteins to the membrane interface. PIPs are mono-, bis-, or trisphosphorylated derivatives of phosphatidylinositol (PI) yielding seven species with different structure and anionic charge. The differential spatial distribution and temporal appearance of PIPs is key to their role in communicating information to target proteins. Selective recognition of PIPs came into play with the discovery that the substrate of protein kinase C termed pleckstrin possessed the first PIP binding region termed the pleckstrin homology (PH) domain. Since the discovery of the PH domain, more than ten PIP binding domains have been identified including PH, ENTH, FYVE, PX, and C2 domains. Representative examples of each of these domains have been thoroughly characterized to understand how they coordinate PIP headgroups in membranes, translocate to specific membrane docking sites in the cell, and function to regulate the activity of their full-length proteins. In addition, a number of novel mechanisms of PIP-mediated membrane association have emerged, such as coincidence detection-specificity for two distinct lipid headgroups. Other PIP-binding domains may also harbor selectivity for a membrane physical property such as charge or membrane curvature. This review summarizes the current understanding of the cellular distribution of PIPs and their molecular interaction with peripheral proteins.
Keywords: C2 domain; FYVE domain; Lipid binding; Membrane binding; PH domain; PI(3)P; PI(3,4,5)P(3); PI(4,5)P(2); Peripheral protein.; Phosphoinsoitide.
Copyright © 2014 Elsevier Ireland Ltd. All rights reserved.
Figures
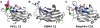
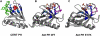
References
-
- Agranoff BW, Bradley RM, Brady RO. The enzymatic synthesis of inositol phosphatide. J. Biol. Chem. 1958;233:1077–1083. - PubMed
-
- Alajlouni R, Drahos KE, Finkielstein CV, Capelluto DG. Lipid-mediated membrane binding properties of Disabled-2. Biochim. Biophys. Acta. 2011;1808:2734–2744. - PubMed
-
- Ankem G, Mitra S, Sun F, Moreno AC, Chutvirasakul B, Azurmend HF, Li L, Capelluto DGS. The C2 domain of Tollip, a toll-like receptor signaling regulator, exhibits broad preference for phosphoinositides. Biochem. J. 2011;435:597–608. - PubMed
-
- Arai Y, Ijuin T, Takenawa T, Becker LE, Takashima S. Excessive expression of synaptojanin in brains with Down syndrome. Brain Development. 2002;24:67–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

