Insights into phagocytosis-coupled activation of pattern recognition receptors and inflammasomes
- PMID: 24556406
- PMCID: PMC3932007
- DOI: 10.1016/j.coi.2013.11.003
Insights into phagocytosis-coupled activation of pattern recognition receptors and inflammasomes
Abstract
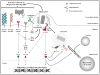
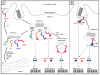
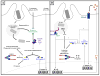
A decade of work shows that the core function of phagocytosis in engulfment and destruction of microorganisms is only a small facet of the full spectrum of roles for phagocytosis in the immune system. The regulation of phagocytosis and its outcomes by inflammatory pattern recognition receptors (PRRs) is now followed by new studies strengthening this concept and adding further complexity to the relationship between phagocytosis and innate immune signaling. Phagocytosis forms the platform for activation of distinct members of the Toll-like receptor family, and even dictates their signaling outcomes. In many cases, phagocytosis is a necessary precedent to the activation of cytosolic PRRs and assembly of canonical and non-canonical inflammasomes, leading to strong pro-inflammatory responses and inflammatory cell death.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

