Design and physicochemical characterisation of novel dissolving polymeric microneedle arrays for transdermal delivery of high dose, low molecular weight drugs
- PMID: 24556420
- PMCID: PMC4034161
- DOI: 10.1016/j.jconrel.2014.02.007
Design and physicochemical characterisation of novel dissolving polymeric microneedle arrays for transdermal delivery of high dose, low molecular weight drugs
Abstract
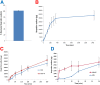
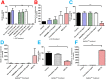
We describe formulation and evaluation of novel dissolving polymeric microneedle (MN) arrays for the facilitated delivery of low molecular weight, high dose drugs. Ibuprofen sodium was used as the model here and was successfully formulated at approximately 50% w/w in the dry state using the copolymer poly(methylvinylether/maleic acid). These MNs were robust and effectively penetrated skin in vitro, dissolving rapidly to deliver the incorporated drug. The delivery of 1.5mg ibuprofen sodium, the theoretical mass of ibuprofen sodium contained within the dry MN alone, was vastly exceeded, indicating extensive delivery of the drug loaded into the baseplates. Indeed in in vitro transdermal delivery studies, approximately 33mg (90%) of the drug initially loaded into the arrays was delivered over 24h. Iontophoresis produced no meaningful increase in delivery. Biocompatibility studies and in vivo rat skin tolerance experiments raised no concerns. The blood plasma ibuprofen sodium concentrations achieved in rats (263μgml(-1) at the 24h time point) were approximately 20 times greater than the human therapeutic plasma level. By simplistic extrapolation of average weights from rats to humans, a MN patch design of no greater than 10cm(2) could cautiously be estimated to deliver therapeutically-relevant concentrations of ibuprofen sodium in humans. This work, therefore, represents a significant progression in exploitation of MN for successful transdermal delivery of a much wider range of drugs.
Keywords: Biocompatibility; Ibuprofen; Microneedles; Transdermal.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures




References
-
- Ito Y., Hirono M., Fukushima K., Sugioka N., Takada K. Two-layered dissolving microneedles formulated with intermediate-acting insulin. Int. J. Pharm. 2012;436:387–393. - PubMed
-
- Liu S., Jin M.N., Quan Y.S., Kamiyama F., Katsumi H., Sakane T., Yamamoto A. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J. Control. Release. 2012;161:933–941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

