Arginase 2 deficiency reduces hyperoxia-mediated retinal neurodegeneration through the regulation of polyamine metabolism
- PMID: 24556690
- PMCID: PMC3944241
- DOI: 10.1038/cddis.2014.23
Arginase 2 deficiency reduces hyperoxia-mediated retinal neurodegeneration through the regulation of polyamine metabolism
Abstract
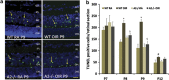
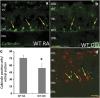
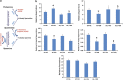
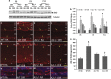
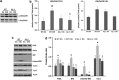
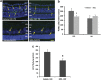
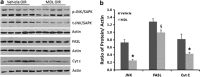
Hyperoxia treatment has been known to induce neuronal and glial death in the developing central nervous system. Retinopathy of prematurity (ROP) is a devastating disease in premature infants and a major cause of childhood vision impairment. Studies indicate that, in addition to vascular injury, retinal neurons are also affected in ROP. Using an oxygen-induced retinopathy (OIR) mouse model for ROP, we have previously shown that deletion of the arginase 2 (A2) significantly reduced neuro-glial injury and improved retinal function. In the current study, we investigated the mechanism of A2 deficiency-mediated neuroprotection in the OIR retina. Hyperoxia treatment has been known to induce neuronal death in neonates. During the hyperoxia phase of OIR, a significant increase in the number of apoptotic cells was observed in the wild-type (WT) OIR retina compared with A2-deficient OIR. Mass spectrometric analysis showed alterations in polyamine metabolism in WT OIR retina. Further, increased expression level of spermine oxidase was observed in WT OIR retina, suggesting increased oxidation of polyamines in OIR retina. These changes were minimal in A2-deficient OIR retina. Treatment using the polyamine oxidase inhibitor, N, N'-bis (2, 3-butadienyl)-1, 4-butanediamine dihydrochloride, significantly improved neuronal survival during OIR treatment. Our data suggest that retinal arginase is involved in the hyperoxia-induced neuronal degeneration in the OIR model, through the regulation of polyamine metabolism.
Figures

References
-
- Felderhoff-Mueser U, Bittigau P, Sifringer M, Jarosz B, Korobowicz E, Mahler L, et al. Oxygen causes cell death in the developing brain. Neurobiol Dis. 2004;17:273–282. - PubMed
-
- Yis U, Kurul SH, Kumral A, Cilaker S, Tugyan K, Genc S, et al. Hyperoxic exposure leads to cell death in the developing brain. Brain Dev. 2008;30:556–562. - PubMed
-
- O'Connor AR, Stephenson T, Johnson A, Tobin MJ, Moseley MJ, Ratib S, et al. Long-term ophthalmic outcome of low birth weight children with and without retinopathy of prematurity. Pediatrics. 2002;109:12–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

