Phosphorylated AKT inhibits the apoptosis induced by DRAM-mediated mitophagy in hepatocellular carcinoma by preventing the translocation of DRAM to mitochondria
- PMID: 24556693
- PMCID: PMC3944266
- DOI: 10.1038/cddis.2014.51
Phosphorylated AKT inhibits the apoptosis induced by DRAM-mediated mitophagy in hepatocellular carcinoma by preventing the translocation of DRAM to mitochondria
Abstract
Increasing autophagy is beneficial for curing hepatocellular carcinoma (HCC). Damage-regulated autophagy modulator (DRAM) was recently reported to induce apoptosis by mediating autophagy. However, the effects of DRAM-mediated autophagy on apoptosis in HCC cells remain unclear. In this study, normal hepatocytes (7702) and HCC cell lines (HepG2, Hep3B and Huh7) were starved for 48 h. Starvation induced apoptosis and autophagy in all cell lines. We determined that starvation also induced DRAM expression and DRAM-mediated autophagy in both normal hepatocytes and HCC cells. However, DRAM-mediated autophagy was involved in apoptosis in normal hepatocytes but not in HCC cells, suggesting that DRAM-mediated autophagy fails to induce apoptosis in hepatoma in response to starvation. Immunoblot and immunofluorescence assays demonstrated that DRAM translocated to mitochondria and induced mitophagy, which led to apoptosis in 7702 cells. In HCC cells, starvation also activated the phosphatidylinositol 3-kinase (PI3K)/AKT pathway, which blocks the translocation of DRAM to mitochondria through the binding of p-AKT to DRAM in the cytoplasm. Inactivation of the PI3K/AKT pathway rescued DRAM translocation to mitochondria; subsequently, mitochondrial DRAM induced apoptosis in HCC cells by mediating mitophagy. Our findings open new avenues for the investigation of the mechanisms of DRAM-mediated autophagy and suggest that promoting DRAM-mediated autophagy together with PI3K/AKT inhibition might be more effective for autophagy-based therapy in hepatoma.
Figures
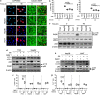

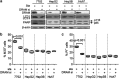




Similar articles
-
MDM2 antagonist can inhibit tumor growth in hepatocellular carcinoma with different types of p53 in vitro.J Gastroenterol Hepatol. 2011 Feb;26(2):371-7. doi: 10.1111/j.1440-1746.2010.06440.x. J Gastroenterol Hepatol. 2011. PMID: 21261729
-
MiR-21 mediates sorafenib resistance of hepatocellular carcinoma cells by inhibiting autophagy via the PTEN/Akt pathway.Oncotarget. 2015 Oct 6;6(30):28867-81. doi: 10.18632/oncotarget.4814. Oncotarget. 2015. PMID: 26311740 Free PMC article.
-
Tanshinone I induces cell apoptosis by reactive oxygen species-mediated endoplasmic reticulum stress and by suppressing p53/DRAM-mediated autophagy in human hepatocellular carcinoma.Artif Cells Nanomed Biotechnol. 2020 Dec;48(1):488-497. doi: 10.1080/21691401.2019.1709862. Artif Cells Nanomed Biotechnol. 2020. PMID: 32013613
-
[EEF1A2 inhibits the p53 function in hepatocellular carcinoma via PI3K/AKT/mTOR-dependent stabilization of MDM4].Pathologe. 2014 Nov;35 Suppl 2:177-84. doi: 10.1007/s00292-014-2007-y. Pathologe. 2014. PMID: 25394965 Review. German.
-
Targeting Akt in Hepatocellular Carcinoma and Its Tumor Microenvironment.Int J Mol Sci. 2021 Feb 11;22(4):1794. doi: 10.3390/ijms22041794. Int J Mol Sci. 2021. PMID: 33670268 Free PMC article. Review.
Cited by
-
Tackling the Cytotoxic Effect of a Marine Polycyclic Quinone-Type Metabolite: Halenaquinone Induces Molt 4 Cells Apoptosis via Oxidative Stress Combined with the Inhibition of HDAC and Topoisomerase Activities.Mar Drugs. 2015 May 20;13(5):3132-53. doi: 10.3390/md13053132. Mar Drugs. 2015. PMID: 26006712 Free PMC article.
-
Proliferation of Lung Epithelial Cells Is Regulated by the Mechanisms of Autophagy Upon Exposure of Soots.Front Cell Dev Biol. 2021 Jul 21;9:662597. doi: 10.3389/fcell.2021.662597. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34368122 Free PMC article.
-
Genes involved in the regulation of different types of autophagy and their participation in cancer pathogenesis.Oncotarget. 2018 Sep 28;9(76):34413-34428. doi: 10.18632/oncotarget.26126. eCollection 2018 Sep 28. Oncotarget. 2018. PMID: 30344951 Free PMC article. Review.
-
DNA damage regulated autophagy modulator 1 recovers the function of apoptosis-stimulating of p53 protein 2 on inducing apoptotic cell death in Huh7.5 cells.Oncol Lett. 2018 Jun;15(6):9333-9338. doi: 10.3892/ol.2018.8453. Epub 2018 Apr 10. Oncol Lett. 2018. PMID: 29844830 Free PMC article.
-
Emerging mechanistic insights of selective autophagy in hepatic diseases.Front Pharmacol. 2023 Mar 16;14:1149809. doi: 10.3389/fphar.2023.1149809. eCollection 2023. Front Pharmacol. 2023. PMID: 37007026 Free PMC article. Review.
References
-
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. - PubMed
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Ding ZB, Shi YH, Zhou J, Qiu SJ, Xu Y, Dai Z, et al. Association of autophagy defect with a malignant phenotype and poor prognosis of hepatocellular carcinoma. Cancer Res. 2008;68:9167–9175. - PubMed
-
- Gao M, Yeh PY, Lu YS, Hsu CH, Chen KF, Lee WC, et al. OSU-03012, a novel celecoxib derivative, induces reactive oxygen species-related autophagy in hepatocellular carcinoma. Cancer Res. 2008;68:9348–9357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

