TPX2: of spindle assembly, DNA damage response, and cancer
- PMID: 24556998
- PMCID: PMC11114040
- DOI: 10.1007/s00018-014-1582-7
TPX2: of spindle assembly, DNA damage response, and cancer
Abstract
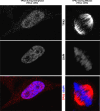
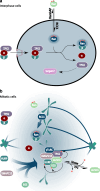
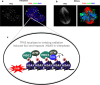
For more than 15 years, TPX2 has been studied as a factor critical for mitosis and spindle assembly. These functions of TPX2 are attributed to its Ran-regulated microtubule-associated protein properties and to its control of the Aurora A kinase. Overexpressed in cancers, TPX2 is being established as marker for the diagnosis and prognosis of malignancies. During interphase, TPX2 resides preferentially in the nucleus where its function had remained elusive until recently. The latest finding that TPX2 plays a role in amplification of the DNA damage response, combined with the characterization of TPX2 knockout mice, open new perspectives to understand the biology of this protein. This review provides an historic overview of the discovery of TPX2 and summarizes its cytoskeletal and signaling roles with relevance to cancer therapies. Finally, the review aims to reconcile discrepancies between the experimental and pathological effects of TPX2 overexpression and advances new roles for compartmentalized TPX2.
Figures

References
-
- Heidebrecht HJ, Buck F, Steinmann J, Sprenger R, Wacker HH, Parwaresch R. p100: a novel proliferation-associated nuclear protein specifically restricted to cell cycle phases S, G2, and M. Blood. 1997;90(1):226–233. - PubMed
-
- Gruss OJ, Wittmann M, Yokoyama H, Pepperkok R, Kufer T, Sillje H, Karsenti E, Mattaj IW, Vernos I. Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nat Cell Biol. 2002;4(11):871–879. - PubMed
-
- Vernos I, Heasman J, Wylie C. Multiple kinesin-like transcripts in Xenopus oocytes. Dev Biol. 1993;157(1):232–239. - PubMed
-
- Boleti H, Karsenti E, Vernos I. Xklp2, a novel Xenopus centrosomal kinesin-like protein required for centrosome separation during mitosis. Cell. 1996;84(1):49–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

