Relative contribution of c1q and apoptotic cell-surface calreticulin to macrophage phagocytosis
- PMID: 24557008
- PMCID: PMC6741593
- DOI: 10.1159/000358834
Relative contribution of c1q and apoptotic cell-surface calreticulin to macrophage phagocytosis
Abstract
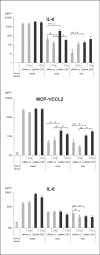
C1q has been shown to recognize apoptotic cells, to enhance their uptake and to modulate cytokine release by phagocytes and thus promote immune tolerance. Surface-exposed calreticulin (CRT), known as a C1q receptor, is also considered to be an early eat-me signal that enhances the phagocytosis of apoptotic cells and is capable of eliciting an immunogenic response. However, the molecular mechanisms that trigger these functions are not clear. We hypothesized that CRT and C1q might act together in these processes. We first showed, by means of fluorescence resonance energy transfer (FRET), that CRT interacts with the C1q globular region at the surface of early apoptotic cells. Next, we pointed out that knockdown of CRT on early apoptotic HeLa cells impairs the enhancement effect of C1q on their uptake by THP-1 monocyte-derived macrophages. Furthermore, a deficiency of CRT induces contrasting effects on cytokine release by THP-1 macrophages, increasing interleukin (IL)-6 and monocyte chemotactic protein 1/CCL2 and decreasing IL-8. Remarkably, these effects were greatly reduced when apoptotic cells were opsonized by C1q, which counterbalanced the effect of the CRT deficiency. These results demonstrate that CRT-C1q interaction is involved in the C1q bridging function and they highlight the particular ability of C1q to control the phagocyte inflammatory status, i.e. by integrating the molecular changes that could occur at the surface of dying cells.
© 2014 S. Karger AG, Basel.
Figures



References
-
- Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J Immunol. 1997;158:4525–4528. - PubMed
-
- Navratil JS, Watkins SC, Wisnieski JJ, Ahearn JM. The globular heads of C1q specifically recognize surface blebs of apoptotic vascular endothelial cells. J Immunol. 2001;166:3231–3239. - PubMed
-
- Nayak A, Ferluga J, Tsolaki AG, Kishore U. The non-classical functions of the classical complement pathway recognition subcomponent C1q. Immunol Lett. 2010;131:139–150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

