Interplay between endothelin and erythropoietin in astroglia: the role in protection against hypoxia
- PMID: 24557580
- PMCID: PMC3958886
- DOI: 10.3390/ijms15022858
Interplay between endothelin and erythropoietin in astroglia: the role in protection against hypoxia
Abstract
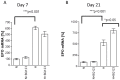
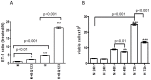
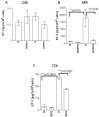
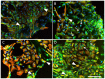
We show that, under in vitro conditions, the vulnerability of astroglia to hypoxia is reflected by alterations in endothelin (ET)-1 release and capacity of erythropoietin (EPO) to regulate ET-1 levels. Exposure of cells to 24 h hypoxia did not induce changes in ET-1 release, while 48-72 h hypoxia resulted in increase of ET-1 release from astrocytes that could be abolished by EPO. The endothelin receptor type A (ETA) antagonist BQ123 increased extracellular levels of ET-1 in human fetal astroglial cell line (SV-FHAS). The survival and proliferation of rat primary astrocytes, neural precursors, and neurons upon hypoxic conditions were increased upon administration of BQ123. Hypoxic injury and aging affected the interaction between the EPO and ET systems. Under hypoxia EPO decreased ET-1 release from astrocytes, while ETA receptor blockade enhanced the expression of EPO mRNA and EPO receptor in culture-aged rat astroglia. The blockade of ETA receptor can increase the availability of ET-1 to the ETB receptor and can potentiate the neuroprotective effects of EPO. Thus, the new therapeutic use of combined administration of EPO and ETA receptor antagonists during hypoxia-associated neurodegenerative disorders of the central nervous system (CNS) can be suggested.
Figures
Similar articles
-
Similar protective effects of BQ-123 and erythropoietin on survival of neural cells and generation of neurons upon hypoxic injury.Eur J Cell Biol. 2005 Nov;84(11):907-13. doi: 10.1016/j.ejcb.2005.07.001. Eur J Cell Biol. 2005. PMID: 16323287
-
The blockade of endothelin A receptor protects astrocytes against hypoxic injury: common effects of BQ-123 anderythropoietin on the rejuvenation of the astrocyte population.Eur J Cell Biol. 2005 Jun;84(5):567-79. doi: 10.1016/j.ejcb.2004.12.030. Eur J Cell Biol. 2005. PMID: 16003910
-
Age-dependent astroglial vulnerability to hypoxia and glutamate: the role for erythropoietin.PLoS One. 2013 Oct 4;8(10):e77182. doi: 10.1371/journal.pone.0077182. eCollection 2013. PLoS One. 2013. PMID: 24124607 Free PMC article.
-
Endothelin in the pulmonary circulation with special reference to hypoxic pulmonary vasoconstriction.Scand Cardiovasc J Suppl. 1997;46:1-40. Scand Cardiovasc J Suppl. 1997. PMID: 9265559 Review.
-
Fount, fate, features, and function of renal erythropoietin-producing cells.Pflugers Arch. 2022 Aug;474(8):783-797. doi: 10.1007/s00424-022-02714-7. Epub 2022 Jun 24. Pflugers Arch. 2022. PMID: 35750861 Free PMC article. Review.
Cited by
-
Effects of erythropoietin and methylprednisolone on AQP4 expression in astrocytes.Mol Med Rep. 2017 Nov;16(5):5924-5930. doi: 10.3892/mmr.2017.7330. Epub 2017 Aug 22. Mol Med Rep. 2017. PMID: 28849166 Free PMC article.
-
Novel applications of trophic factors, Wnt and WISP for neuronal repair and regeneration in metabolic disease.Neural Regen Res. 2015 Apr;10(4):518-28. doi: 10.4103/1673-5374.155427. Neural Regen Res. 2015. PMID: 26170801 Free PMC article. Review.
-
Regeneration in the nervous system with erythropoietin.Front Biosci (Landmark Ed). 2016 Jan;21(3):561-596. doi: 10.2741/4408. Front Biosci (Landmark Ed). 2016. PMID: 26549969 Free PMC article.
-
Targeting Endothelin in Alzheimer's Disease: A Promising Therapeutic Approach.Biomed Res Int. 2021 Sep 6;2021:7396580. doi: 10.1155/2021/7396580. eCollection 2021. Biomed Res Int. 2021. PMID: 34532504 Free PMC article. Review.
-
Neuroprotective, Neurogenic, and Amyloid Beta Reducing Effect of a Novel Alpha 2-Adrenoblocker, Mesedin, on Astroglia and Neuronal Progenitors upon Hypoxia and Glutamate Exposure.Int J Mol Sci. 2017 Dec 21;19(1):9. doi: 10.3390/ijms19010009. Int J Mol Sci. 2017. PMID: 29267189 Free PMC article.
References
-
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;32:411–415. - PubMed
-
- Gandhi C.R., Nemoto E.M., Watkins S.C., Subbotin V.M. An endothelin receptor antagonist TAK-044 ameliorates carbon tetrachloride-induced acute liver injury and portal hypertension in rats. Liver. 1998;18:39–48. - PubMed
-
- Sakurai T., Yanagisawa M., Inoue A., Ryan U.S., Kimura S., Mitsui I., Goto K., Masaki T. cDNA cloning, sequence analysis and tissue distribution of rat preproendothelin-1 mRNA. Biochem. Biophys. Res. Commun. 1991;175:44–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

