Tracheal occlusion-evoked respiratory load compensation and inhibitory neurotransmitter expression in rats
- PMID: 24557797
- PMCID: PMC4035788
- DOI: 10.1152/japplphysiol.01256.2013
Tracheal occlusion-evoked respiratory load compensation and inhibitory neurotransmitter expression in rats
Abstract
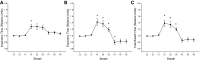
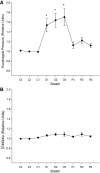
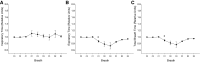
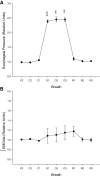
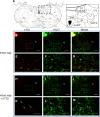
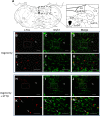
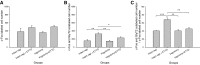
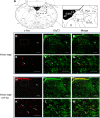
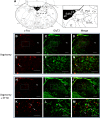
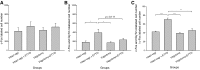
Respiratory load compensation is a sensory-motor reflex generated in the brain stem respiratory neural network. The nucleus of the solitary tract (NTS) is thought to be the primary structure to process the respiratory load-related afferent activity and contribute to the modification of the breathing pattern by sending efferent projections to other structures in the brain stem respiratory neural network. The sensory pathway and motor responses of respiratory load compensation have been studied extensively; however, the mechanism of neurogenesis of load compensation is still unknown. A variety of studies has shown that inhibitory interconnections among the brain stem respiratory groups play critical roles for the genesis of respiratory rhythm and pattern. The purpose of this study was to examine whether inhibitory glycinergic neurons in the NTS were activated by external and transient tracheal occlusions (ETTO) in anesthetized animals. The results showed that ETTO produced load compensation responses with increased inspiratory, expiratory, and total breath time, as well as elevated activation of inhibitory glycinergic neurons in the caudal NTS (cNTS) and intermediate NTS (iNTS). Vagotomized animals receiving transient respiratory loads did not exhibit these load compensation responses. In addition, vagotomy significantly reduced the activation of inhibitory glycinergic neurons in the cNTS and iNTS. The results suggest that these activated inhibitory glycinergic neurons in the NTS might be essential for the neurogenesis of load compensation responses in anesthetized animals.
Keywords: NTS; glycinergic neurons; load compensation; tracheal occlusion; vagotomy.
Figures

References
-
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev 75: 1–45, 1995 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

