Human adult white matter progenitor cells are multipotent neuroprogenitors similar to adult hippocampal progenitors
- PMID: 24558163
- PMCID: PMC3973711
- DOI: 10.5966/sctm.2013-0117
Human adult white matter progenitor cells are multipotent neuroprogenitors similar to adult hippocampal progenitors
Abstract
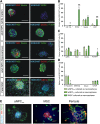
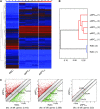
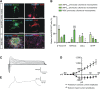
Adult neural progenitor cells (aNPC) are a potential autologous cell source for cell replacement in neurologic diseases or for cell-based gene therapy of neurometabolic diseases. Easy accessibility, long-term expandability, and detailed characterization of neural progenitor cell (NPC) properties are important requisites for their future translational/clinical applications. aNPC can be isolated from different regions of the adult human brain, including the accessible subcortical white matter (aNPCWM), but systematic studies comparing long-term expanded aNPCWM with aNPC from neurogenic brain regions are not available. Freshly isolated cells from subcortical white matter and hippocampus expressed oligodendrocyte progenitor cell markers such as A2B5, neuron-glial antigen 2 (NG2), and oligodendrocyte transcription factor 2 (OLIG2) in ∼20% of cells but no neural stem cell (NSC) markers such as CD133 (Prominin1), Nestin, SOX2, or PAX6. The epidermal growth factor receptor protein was expressed in 18% of aNPCWM and 7% of hippocampal aNPC (aNPCHIP), but only a small fraction of cells, 1 of 694 cells from white matter and 1 of 1,331 hippocampal cells, was able to generate neurospheres. Studies comparing subcortical aNPCWM with their hippocampal counterparts showed that both NPC types expressed mainly markers of glial origin such as NG2, A2B5, and OLIG2, and the NSC/NPC marker Nestin, but no pericyte markers. Both NPC types were able to produce neurons, astrocytes, and oligodendrocytes in amounts comparable to fetal NSC. Whole transcriptome analyses confirmed the strong similarity of aNPCWM to aNPCHIP. Our data show that aNPCWM are multipotent NPC with long-term expandability similar to NPC from hippocampus, making them a more easily accessible source for possible autologous NPC-based treatment strategies.
Keywords: Fetal neural stem cells; Hippocampus; Human adult brain; Neural progenitor cells; White matter.
Figures


Similar articles
-
A2B5 Expression in Central Nervous System and Gliomas.Int J Mol Sci. 2022 Apr 23;23(9):4670. doi: 10.3390/ijms23094670. Int J Mol Sci. 2022. PMID: 35563061 Free PMC article. Review.
-
Identification and characterization of a new source of adult human neural progenitors.Cell Death Dis. 2017 Aug 10;8(8):e2991. doi: 10.1038/cddis.2017.368. Cell Death Dis. 2017. PMID: 28796246 Free PMC article.
-
CD133/CD140a-based isolation of distinct human multipotent neural progenitor cells and oligodendrocyte progenitor cells.Stem Cells Dev. 2013 Aug 1;22(15):2121-31. doi: 10.1089/scd.2013.0003. Epub 2013 Apr 30. Stem Cells Dev. 2013. PMID: 23488628 Free PMC article.
-
SOX2+ cell population from normal human brain white matter is able to generate mature oligodendrocytes.PLoS One. 2014 Jun 5;9(6):e99253. doi: 10.1371/journal.pone.0099253. eCollection 2014. PLoS One. 2014. PMID: 24901457 Free PMC article.
-
[Glial cells function as neural stem cells and progenitor cells].Sheng Li Xue Bao. 2017 Apr 25;69(2):207-217. Sheng Li Xue Bao. 2017. PMID: 28435980 Review. Chinese.
Cited by
-
A2B5 Expression in Central Nervous System and Gliomas.Int J Mol Sci. 2022 Apr 23;23(9):4670. doi: 10.3390/ijms23094670. Int J Mol Sci. 2022. PMID: 35563061 Free PMC article. Review.
-
Identification and characterization of a new source of adult human neural progenitors.Cell Death Dis. 2017 Aug 10;8(8):e2991. doi: 10.1038/cddis.2017.368. Cell Death Dis. 2017. PMID: 28796246 Free PMC article.
-
Glial restricted precursor cells in central nervous system disorders: Current applications and future perspectives.Glia. 2021 Mar;69(3):513-531. doi: 10.1002/glia.23922. Epub 2020 Oct 14. Glia. 2021. PMID: 33052610 Free PMC article. Review.
-
Neuroregeneration and functional recovery after stroke: advancing neural stem cell therapy toward clinical application.Neural Regen Res. 2021 Jan;16(1):80-92. doi: 10.4103/1673-5374.286955. Neural Regen Res. 2021. PMID: 32788451 Free PMC article.
-
Distinctive Patterns of Seizure-Related White Matter Alterations in Right and Left Temporal Lobe Epilepsy.Front Neurol. 2019 Oct 1;10:986. doi: 10.3389/fneur.2019.00986. eCollection 2019. Front Neurol. 2019. PMID: 31632330 Free PMC article.
References
-
- Hermann A, Maisel M, Liebau S, et al. Mesodermal cell types induce neurogenesis from adult human hippocampal progenitor cells. J Neurochem. 2006;98:629–640. - PubMed
-
- Coras R, Siebzehnrubl FA, Pauli E, et al. Low proliferation and differentiation capacities of adult hippocampal stem cells correlate with memory dysfunction in humans. Brain. 2010;133:3359–3372. - PubMed
-
- Kukekov VG, Laywell ED, Suslov O, et al. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999;156:333–344. - PubMed
-
- Nunes MC, Roy NS, Keyoung HM, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–447. - PubMed
-
- Scolding NJ, Rayner PJ, Compston DA. Identification of A2B5-positive putative oligodendrocyte progenitor cells and A2B5-positive astrocytes in adult human white matter. Neuroscience. 1999;89:1–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

