Progressive dysfunction of the retinal pigment epithelium and retina due to increased VEGF-A levels
- PMID: 24558195
- PMCID: PMC3986839
- DOI: 10.1096/fj.13-248021
Progressive dysfunction of the retinal pigment epithelium and retina due to increased VEGF-A levels
Abstract
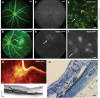
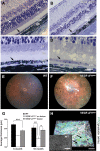
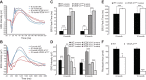
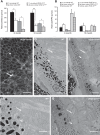
Patients with nonexudative ("dry") age-related macular degeneration (AMD) frequently also develop neovascular ("wet") AMD, suggesting a common pathomechanism. Increased vascular endothelial growth factor A (VEGF-A) has been implicated in the pathogenesis of choroidal neovascularization (CNV) in neovascular AMD, while its role in nonexudative AMD that manifests with progressive retinal pigment epithelium (RPE) and photoreceptor degeneration is not well defined. Mice with overall increased VEGF-A levels develop progressive morphological features of both forms of AMD, suggesting that an increase in VEGF-A has a direct age-dependent adverse effect on RPE and photoreceptor function independently of its CNV-promoting proangiogenic effect. Here we provide evidence for this hypothesis and show that morphological RPE abnormalities and retinal thinning in mice with increased VEGF-A levels correlate with progressive age-dependent attenuation of visual function with abnormal electroretinograms and reduced retinal rhodopsin levels. Retinoid profiling revealed a progressive reduction of 11-cis and all-trans retinal in the retinas of these mice, consistent with an impaired retinoid transport between the RPE and photoreceptors. These findings suggest that increased VEGF-A leads to an age-dependent RPE and retinal dysfunction that occurs also at sites where no CNV lesions form. The data support a central role of increased VEGF-A not only in the pathogenesis of neovascular but also of nonexudative AMD.
Keywords: age-related macular degeneration; choroidal neovascularization; retinoids; visual cycle.
Figures

References
-
- Friedman D. S., O'Colmain B. J., Munoz B., Tomany S. C., McCarty C., de Jong P. T., Nemesure B., Mitchell P., Kempen J. (2004) Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 122, 564–572 - PubMed
-
- Van Leeuwen R., Klaver C. C., Vingerling J. R., Hofman A., de Jong P. T. (2003) The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam study. Arch. Ophthalmol. 121, 519–526 - PubMed
-
- Bird A. C., Bressler N. M., Bressler S. B., Chisholm I. H., Coscas G., Davis M. D., de Jong P. T., Klaver C. C., Klein B. E., Klein R., et al. (1995) An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv. Ophthalmol. 39, 367–374 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

