A systematic review of biomarkers for disease progression in Alzheimer's disease
- PMID: 24558437
- PMCID: PMC3928315
- DOI: 10.1371/journal.pone.0088854
A systematic review of biomarkers for disease progression in Alzheimer's disease
Erratum in
- PLoS One. 2014;9(5):e97960
Abstract
Background: Using surrogate biomarkers for disease progression as endpoints in neuroprotective clinical trials may help differentiate symptomatic effects of potential neuroprotective agents from true slowing of the neurodegenerative process. A systematic review was undertaken to determine what biomarkers for disease progression in Alzheimer's disease exist and how well they perform.
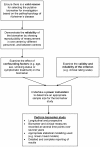
Methods: MEDLINE and Embase (1950-2011) were searched using five search strategies. Abstracts were assessed to identify papers meriting review in full. Studies of participants with probable Alzheimer's disease diagnosed by formal criteria were included. We made no restriction on age, disease duration, or drug treatment. We only included studies with a longitudinal design, in which the putative biomarker and clinical measure were both measured at least twice, as this is the only appropriate study design to use when developing a disease progression biomarker. We included studies which attempted to draw associations between the changes over time in the biomarker used to investigate disease progression and a clinical measure of disease progression.
Results: Fifty-nine studies were finally included. The commonest biomarker modality examined was brain MRI (17/59, 29% of included studies). Median follow-up in included studies was only 1.0 (IQR 0.8-1.7) year and most studies only measured the putative biomarker and clinical measure twice. Included studies were generally of poor quality with small numbers of participants (median 31 (IQR 17 to 64)), applied excessively restrictive study entry criteria, had flawed methodologies and conducted overly simplistic statistical analyses without adjusting for confounding factors.
Conclusions: We found insufficient evidence to recommend the use of any biomarker as an outcome measure for disease progression in Alzheimer's disease trials. However, further investigation into the efficacy of using MRI measurements of ventricular volume and whole brain volume appeared to be merited. A provisional 'roadmap' to improve the quality of future disease progression biomarker studies is presented.
Conflict of interest statement
Figures

References
-
- Knapp M, Prince M, Albanese E, Banjeree S, Dhanasiri S, et al.. (2007) A report to the Alzheimer's Society on the prevalance and economic cost of dementia in the UK produced by King's College London and the London School of Economics. London: Alzheimer's Society.
-
- Knopman D (2006) Finding potent drugs for Alzheimer's disease is more important than proving the drugs are disease modifying. Alzheimers Dement 2: 147–149. - PubMed
-
- Temple RJ (1995) A regulatory authority's opinion about surrogate endpoints. In: Nimmo W, Tucker G, editors. Clinical Measurement in Drug Evaluation. New York: J. Wiley.
-
- Biomarkers Definitions Working Group (2001) Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69: 89–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

