Defining the interactions and role of DCAF1/VPRBP in the DDB1-cullin4A E3 ubiquitin ligase complex engaged by HIV-1 Vpr to induce a G2 cell cycle arrest
- PMID: 24558487
- PMCID: PMC3928422
- DOI: 10.1371/journal.pone.0089195
Defining the interactions and role of DCAF1/VPRBP in the DDB1-cullin4A E3 ubiquitin ligase complex engaged by HIV-1 Vpr to induce a G2 cell cycle arrest
Abstract
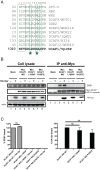
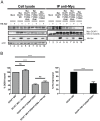
HIV viral protein R (Vpr) induces a cell cycle arrest at the G2/M phase by activating the ATR DNA damage/replication stress signalling pathway through engagement of the DDB1-CUL4A-DCAF1 E3 ubiquitin ligase via a direct binding to the substrate specificity receptor DCAF1. Since no high resolution structures of the DDB1-DCAF1-Vpr substrate recognition module currently exist, we used a mutagenesis approach to better define motifs in DCAF1 that are crucial for Vpr and DDB1 binding. Herein, we show that the minimal domain of DCAF1 that retained the ability to bind Vpr and DDB1 was mapped to residues 1041 to 1393 (DCAF1 WD). Mutagenic analyses identified an α-helical H-box motif and F/YxxF/Y motifs located in the N-terminal domain of DCAF1 WD that are involved in exclusive binding to DDB1. While we could not identify elements specifically involved in Vpr binding, overall, the mutagenesis data suggest that the predicted β-propeller conformation of DCAF1 is likely to be critical for Vpr association. Importantly, we provide evidence that binding of Vpr to DCAF1 appears to modulate the formation of a DDB1/DCAF1 complex. Lastly, we show that expression of DCAF1 WD in the absence of endogenous DCAF1 was not sufficient to enable Vpr-mediated G2 arrest activity. Overall, our results reveal that Vpr and DDB1 binding on DCAF1 can be genetically separated and further suggest that DCAF1 contains determinants in addition to the Vpr and DDB1 minimal binding domain, which are required for Vpr to enable the induction of a G2 arrest.
Conflict of interest statement
Figures




Similar articles
-
Interactions with DCAF1 and DDB1 in the CRL4 E3 ubiquitin ligase are required for Vpr-mediated G2 arrest.Virol J. 2014 Jun 9;11:108. doi: 10.1186/1743-422X-11-108. Virol J. 2014. PMID: 24912982 Free PMC article.
-
HIV-1 Vpr Protein Enhances Proteasomal Degradation of MCM10 DNA Replication Factor through the Cul4-DDB1[VprBP] E3 Ubiquitin Ligase to Induce G2/M Cell Cycle Arrest.J Biol Chem. 2015 Jul 10;290(28):17380-9. doi: 10.1074/jbc.M115.641522. Epub 2015 Jun 1. J Biol Chem. 2015. PMID: 26032416 Free PMC article.
-
HIV-1 Vpr-mediated G2 arrest involves the DDB1-CUL4AVPRBP E3 ubiquitin ligase.PLoS Pathog. 2007 Jul;3(7):e85. doi: 10.1371/journal.ppat.0030085. PLoS Pathog. 2007. PMID: 17630831 Free PMC article.
-
Lentivirus Vpr and Vpx accessory proteins usurp the cullin4-DDB1 (DCAF1) E3 ubiquitin ligase.Curr Opin Virol. 2012 Dec;2(6):755-63. doi: 10.1016/j.coviro.2012.09.010. Epub 2012 Oct 10. Curr Opin Virol. 2012. PMID: 23062609 Free PMC article. Review.
-
The functions of the HIV1 protein Vpr and its action through the DCAF1.DDB1.Cullin4 ubiquitin ligase.Cytokine. 2010 Jul;51(1):1-9. doi: 10.1016/j.cyto.2010.02.018. Epub 2010 Mar 27. Cytokine. 2010. PMID: 20347598 Free PMC article. Review.
Cited by
-
AKT Phosphorylates FAM13A and Promotes Its Degradation via CUL4A/DDB1/DCAF1 E3 Complex.Am J Respir Cell Mol Biol. 2023 May;68(5):577-590. doi: 10.1165/rcmb.2022-0362OC. Am J Respir Cell Mol Biol. 2023. PMID: 36749583 Free PMC article.
-
DCAF1 (VprBP): emerging physiological roles for a unique dual-service E3 ubiquitin ligase substrate receptor.J Mol Cell Biol. 2019 Sep 19;11(9):725-735. doi: 10.1093/jmcb/mjy085. J Mol Cell Biol. 2019. PMID: 30590706 Free PMC article.
-
Defining the roles for Vpr in HIV-1-associated neuropathogenesis.J Neurovirol. 2016 Aug;22(4):403-15. doi: 10.1007/s13365-016-0436-5. Epub 2016 Apr 7. J Neurovirol. 2016. PMID: 27056720 Free PMC article. Review.
-
miRNA-1236 inhibits HIV-1 infection of monocytes by repressing translation of cellular factor VprBP.PLoS One. 2014 Jun 16;9(6):e99535. doi: 10.1371/journal.pone.0099535. eCollection 2014. PLoS One. 2014. PMID: 24932481 Free PMC article.
-
Vpr Is a VIP: HIV Vpr and Infected Macrophages Promote Viral Pathogenesis.Viruses. 2020 Jul 27;12(8):809. doi: 10.3390/v12080809. Viruses. 2020. PMID: 32726944 Free PMC article. Review.
References
-
- Wen X, Duus KM, Friedrich TD, de Noronha CM (2007) The HIV1 protein Vpr acts to promote G2 cell cycle arrest by engaging a DDB1 and Cullin4A-containing ubiquitin ligase complex using VprBP/DCAF1 as an adaptor. J Biol Chem 282: 27046–27057. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

