Accelerated fraction radiotherapy with capecitabine as neoadjuvant therapy for borderline resectable pancreatic cancer
- PMID: 24558510
- PMCID: PMC3924761
Accelerated fraction radiotherapy with capecitabine as neoadjuvant therapy for borderline resectable pancreatic cancer
Abstract
Background: A standard neoadjuvant regimen has not been defined for borderline resectable (BR) pancreatic cancer. This phase II trial was designed to determine the safety of accelerated fraction radiotherapy (AFRT) with capecitabine in patients with BR pancreatic cancer.
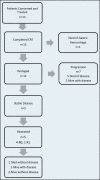
Methods: The patients had newly diagnosed BR adenocarcinoma of the pancreas and normal organ function. Intensity-modulated (n = 11) or 3D conformal (n = 2) radiotherapy was given to a dose of 50 Gy in 2.5-Gy fractions with capecitabine 825 mg/m(2) twice on radiation days. The primary outcome was the frequency of severe treatment-related adverse events (AEs). The study was stopped before planned interim analysis because of 2 severe (grades 4 and 5) gastric ulcerations.
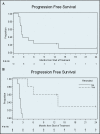
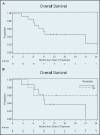
Results: Thirteen patients were enrolled with a median age of 66 years. All patients completed treatment. Seven (54%) experienced grade 3+ treatment-related AEs. Severe gastric ulceration occurred in 2 patients despite receipt of ≥43 Gy to only 1% (2-3 cm(3)) of the stomach. Lymphopenia (n = 7) was the only other severe AE that occurred in >1 patient. In 7 of the 13 patients, disease had progressed outside the pancreas at restaging. Five of the 13 underwent resection, and all had >10% viable tumor. Median progression-free survival (PFS) was 2.4 months (95% CI 1.9-5.9), and median survival was 9.1 months (95% CI 5.9-not reached). Among those who underwent resection, median PFS was 13.0 months (95% CI 4.4-not reached). Median survival was not reached.
Conclusions: Given the limited efficacy signal and severe gastric ulcerations, we do not recommend this regimen for pancreatic cancer. We also do not recommend the use of high doses per fraction outside a clinical trial.
Figures
References
-
- Winter JM, Cameron JL, Campbell KA, et al. : 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 10:1199–1210, 2006; discussion 1210-1211 - PubMed
-
- Oettle H, Post S, Neuhaus P, et al. : Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 297:267–277, 2007 - PubMed
-
- Neoptolemos JP, Stocken DD, Bassi C, et al. : Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 304:1073–81, 2010 - PubMed
-
- Kalser MH, Ellenberg SS: Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 120:899–903, 1985 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
