Copper/TEMPO-Catalyzed Aerobic Alcohol Oxidation: Mechanistic Assessment of Different Catalyst Systems
- PMID: 24558634
- PMCID: PMC3925889
- DOI: 10.1021/cs400689a
Copper/TEMPO-Catalyzed Aerobic Alcohol Oxidation: Mechanistic Assessment of Different Catalyst Systems
Abstract
Combinations of homogeneous Cu salts and TEMPO have emerged as practical and efficient catalysts for the aerobic oxidation of alcohols. Several closely related catalyst systems have been reported, which differ in the identity of the solvent, the presence of 2,2'-bipyridine as a ligand, the identity of basic additives, and the oxidation state of the Cu source. These changes have a significant influence on the reaction rates, yields, and substrate scope. In this report, we probe the mechanistic basis for differences among four different Cu/TEMPO catalyst systems and elucidate the features that contribute to efficient oxidation of aliphatic alcohols.
Keywords: TEMPO; aerobic; alcohol oxidation; copper; kinetics; mechanism.
Figures
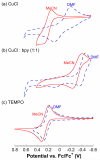
 ) and without (
) and without ( ) bpy in DMF. Standard conditions: 0.4 M CyCH2OH, 0.04 M TEMPO, 0.04 M CuCl, 0.04 M bpy, 600 torr O2, 27 °C.
) bpy in DMF. Standard conditions: 0.4 M CyCH2OH, 0.04 M TEMPO, 0.04 M CuCl, 0.04 M bpy, 600 torr O2, 27 °C.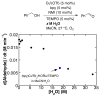
 ) included for comparison.
) included for comparison.
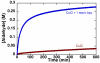
 ) and [(bpy)Cu(OH)]2(OTf)2. (
) and [(bpy)Cu(OH)]2(OTf)2. ( ). Data were obtained by monitoring pressure changes during catalytic turnover. Reaction conditions: 10 mM (bpy)Cu, 10 mM TEMPO, 20 mM NMI: 0.2 M CyCH2 OH, 1 atm O2, 2.5 mL MeCN, 27 °C.
). Data were obtained by monitoring pressure changes during catalytic turnover. Reaction conditions: 10 mM (bpy)Cu, 10 mM TEMPO, 20 mM NMI: 0.2 M CyCH2 OH, 1 atm O2, 2.5 mL MeCN, 27 °C.

 ) is included in A for comparison. Rates were obtained by monitoring pressure changes during catalytic turnover. Standard reaction conditions: 10 mM CuI(OTf), 10 mM bpy, 20 mM NMI, 10 mM TEMPO, 0.2 M alcohol.
) is included in A for comparison. Rates were obtained by monitoring pressure changes during catalytic turnover. Standard reaction conditions: 10 mM CuI(OTf), 10 mM bpy, 20 mM NMI, 10 mM TEMPO, 0.2 M alcohol.

Similar articles
-
Mechanism of copper(I)/TEMPO-catalyzed aerobic alcohol oxidation.J Am Chem Soc. 2013 Feb 13;135(6):2357-67. doi: 10.1021/ja3117203. Epub 2013 Jan 31. J Am Chem Soc. 2013. PMID: 23317450 Free PMC article.
-
Copper(I)/ABNO-catalyzed aerobic alcohol oxidation: alleviating steric and electronic constraints of Cu/TEMPO catalyst systems.J Am Chem Soc. 2013 Oct 23;135(42):15742-5. doi: 10.1021/ja409241h. Epub 2013 Oct 15. J Am Chem Soc. 2013. PMID: 24128057 Free PMC article.
-
Spectroscopic Characterization of a Reactive [Cu2 (μ-OH)2 ]2+ Intermediate in Cu/TEMPO Catalyzed Aerobic Alcohol Oxidation Reaction.Angew Chem Int Ed Engl. 2021 Oct 11;60(42):23018-23024. doi: 10.1002/anie.202108442. Epub 2021 Sep 8. Angew Chem Int Ed Engl. 2021. PMID: 34309168 Free PMC article.
-
Practical aerobic oxidations of alcohols and amines with homogeneous copper/TEMPO and related catalyst systems.Angew Chem Int Ed Engl. 2014 Aug 18;53(34):8824-38. doi: 10.1002/anie.201403110. Epub 2014 Jul 7. Angew Chem Int Ed Engl. 2014. PMID: 25044821 Free PMC article. Review.
-
Ligand-Promoted Palladium-Catalyzed Aerobic Oxidation Reactions.Chem Rev. 2018 Mar 14;118(5):2636-2679. doi: 10.1021/acs.chemrev.7b00334. Epub 2017 Oct 4. Chem Rev. 2018. PMID: 28975795 Review.
Cited by
-
Electrochemical Performance of ABNO for Oxidation of Secondary Alcohols in Acetonitrile Solution.Molecules. 2018 Dec 28;24(1):100. doi: 10.3390/molecules24010100. Molecules. 2018. PMID: 30597882 Free PMC article.
-
Multivariate Analysis of Coupled Operando EPR/XANES/EXAFS/UV-Vis/ATR-IR Spectroscopy: A New Dimension for Mechanistic Studies of Catalytic Gas-Liquid Phase Reactions.Chemistry. 2020 Jun 10;26(33):7395-7404. doi: 10.1002/chem.202000436. Epub 2020 May 14. Chemistry. 2020. PMID: 32118340 Free PMC article.
-
CuCl/TMEDA/nor-AZADO-catalyzed aerobic oxidative acylation of amides with alcohols to produce imides.Chem Sci. 2018 May 7;9(21):4756-4768. doi: 10.1039/c8sc01410h. eCollection 2018 Jun 7. Chem Sci. 2018. PMID: 29910926 Free PMC article.
-
Copper-Nitroxyl-Catalyzed α-Oxygenation of Cyclic Secondary Amines Including Application to Late-Stage Functionalization.J Am Chem Soc. 2024 May 29;146(21):14439-14444. doi: 10.1021/jacs.4c04359. Epub 2024 May 14. J Am Chem Soc. 2024. PMID: 38743876 Free PMC article.
-
Soluble/MOF-Supported Palladium Single Atoms Catalyze the Ligand-, Additive-, and Solvent-Free Aerobic Oxidation of Benzyl Alcohols to Benzoic Acids.J Am Chem Soc. 2021 Feb 17;143(6):2581-2592. doi: 10.1021/jacs.0c12367. Epub 2021 Feb 4. J Am Chem Soc. 2021. PMID: 33535758 Free PMC article.
References
-
- Tojo G, Fernández M. In: Oxidation of Alcohols to Aldehydes and Ketones. Tojo G, editor. Springer; New York: 2010.
-
- Arends IWCE, Sheldon RA. In: Modern Oxidation Methods. Bäckvall J-E, editor. Wiley-VCH Verlag Gmb & Co.; Weinheim: 2004. pp. 83–118.
- Sheldon RA, Arends IWCE, ten Brink G-J, Dijksman A. Acc. Chem. Res. 2002;35:774–781. - PubMed
- Zhan BZ, Thompson A. Tetrahedron. 2004;60:2917–2935.
- Mallat T, Baiker A. Chem. Rev. 2004;104:3037–3058. - PubMed
- Stahl SS. Angew. Chem., Int. Ed. 2004;43:3400–3420. - PubMed
- Markó IE, Giles PR, Tsukazaki M, Chellé-Regnaut I, Gautier A, Dumeunier R, Philippart F, Doda K, Mutonkole J-L, Brown SM, Urch CJ. Adv. Inorg. Chem. 2004;56:211–240.
- Schultz MJ, Sigman MS. Tetrahedron. 2006;62:8227–8241.
- Sigman MS, Jensen DR. Acc. Chem. Res. 2006;39:221–229. - PubMed
- Karimi B, Zamani A. J Iran Chem Soc. 2008;5:S1–S20.
- Matsumoto T, Ueno M, Wang N, Kobayashi S. Chem-Asian J. 2008;3:196–214. - PubMed
- Parmeggiani C, Cardona F. Green Chem. 2012;14:547–564.
-
- Semmelhack MF, Schmid CR, Cortés DA, Chou CS. J. Am. Chem. Soc. 1984;106:3374–3376.
-
- Gamez P, Arends IWCE, Reedijk J, Sheldon RA. Chem. Commun. 2003:2414–2415. - PubMed
- Gamez P, Arends IWCE, Sheldon RA, Reedijk J. Adv. Synth. Catal. 2004;346:805–811.
-
- Kumpulainen ETT, Koskinen AMP. Chem. Eur. J. 2009;15:10901–10911. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
