A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease
- PMID: 24559284
- PMCID: PMC3990004
- DOI: 10.1021/nn405033r
A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease
Abstract
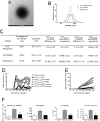
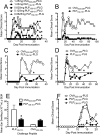
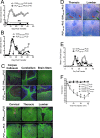
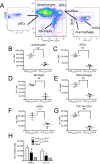
Targeted immune tolerance is a coveted therapy for the treatment of a variety of autoimmune diseases, as current treatment options often involve nonspecific immunosuppression. Intravenous (iv) infusion of apoptotic syngeneic splenocytes linked with peptide or protein autoantigens using ethylene carbodiimide (ECDI) has been demonstrated to be an effective method for inducing peripheral, antigen-specific tolerance for treatment of autoimmune disease. Here, we show the ability of biodegradable poly(lactic-co-glycolic acid) (PLG) nanoparticles to function as a safe, cost-effective, and highly efficient alternative to cellular carriers for the induction of antigen-specific T cell tolerance. We describe the formulation of tolerogenic PLG particles and demonstrate that administration of myelin antigen-coupled particles both prevented and treated relapsing-remitting experimental autoimmune encephalomyelitis (R-EAE), a CD4 T cell-mediated mouse model of multiple sclerosis (MS). PLG particles made on-site with surfactant modifications surpass the efficacy of commercially available particles in their ability to couple peptide and to prevent disease induction. Most importantly, myelin antigen-coupled PLG nanoparticles are able to significantly ameliorate ongoing disease and subsequent relapses when administered at onset or at peak of acute disease, and minimize epitope spreading when administered during disease remission. Therapeutic treatment results in significantly reduced CNS infiltration of encephalitogenic Th1 (IFN-γ) and Th17 (IL-17a) cells as well as inflammatory monocytes/macrophages. Together, these data describe a platform for antigen display that is safe, low-cost, and highly effective at inducing antigen-specific T cell tolerance. The development of such a platform carries broad implications for the treatment of a variety of immune-mediated diseases.
Figures

References
-
- American Autoimmune Related Diseases Association The Cost Burden of Autoimmune Disease: The Latest Front in the War on Healthcare Spending; AARDA: Eastpointe, MI, 2011. http://www.aarda.org/pdf/cbad.pdf.
-
- Chatenoud L.; Primo J.; Bach J. F. CD3 Antibody-Induced Dominant Self Tolerance in Overtly Diabetic Nod Mice. J. Immunol. 1997, 158, 2947–2954. - PubMed
-
- Belghith M.; Bluestone J. A.; Barriot S.; Megret J.; Bach J. F.; Chatenoud L. Tgf-Beta-Dependent Mechanisms Mediate Restoration of Self-Tolerance Induced by Antibodies to CD3 in Overt Autoimmune Diabetes. Nat. Med. 2003, 9, 1202–1208. - PubMed
-
- Kohm A. P.; Williams J. S.; Bickford A. L.; McMahon J. S.; Chatenoud L.; Bach J. F.; Bluestone J. A.; Miller S. D. Treatment with Nonmitogenic Anti-CD3Monoclonal Antibody Induces CD4+ T Cell Unresponsiveness and Functional Reversal of Established Experimental Autoimmune Encephalomyelitis. J. Immunol. 2005, 174, 4525–4534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

