Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response
- PMID: 24559482
- PMCID: PMC4061541
- DOI: 10.1164/rccm.201306-1043OC
Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response
Abstract
Rationale: Aging is characterized by functional impairment and reduced capacity to respond appropriately to environmental stimuli and injury. With age, there is an increase in the incidence and severity of chronic and acute lung diseases. However, the relationship between age and the lung's reduced ability to repair is far from established and necessitates further research in the field.
Objectives: Little is currently known about age-related phenomena in mesenchymal stem cells (MSCs). On account of their ability to protect the endothelium and the alveolar epithelium through multiple paracrine mechanisms, we looked for adverse effects that aging might cause in MSC biology. Such age-related changes might partly account for the increased susceptibility of the aging lung to injury.
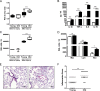
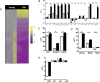
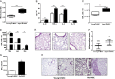
Measurements and main results: We demonstrated that old mice have more inflammation in response to acute lung injury. To investigate the causes, we compared the global gene expression of aged and young bone marrow-derived MSCs (B-MSCs). Our results revealed that the expression levels of inflammatory response genes depended on the age of the B-MSCs. We demonstrated that the age-dependent decrease in expression of several cytokine and chemokine receptors is important for the migration and activation of B-MSCs. Finally, we showed by adoptive transfer of aged B-MSCs to young endotoxemic mice that aged cells lacked the antiinflammatory protective effect of their young counterparts.
Conclusions: Taken together, the decreased expression of cytokine and chemokine receptors in aged B-MSCs compromises their protective role by perturbing the potential of B-MSCs to become activated and mobilize to the site of injury.
Figures




References
-
- Faner R, Rojas M, Macnee W, Agustí A. Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:306–313. - PubMed
-
- Selman M, Rojas M, Mora AL, Pardo A. Aging and interstitial lung diseases: unraveling an old forgotten player in the pathogenesis of lung fibrosis. Semin Respir Crit Care Med. 2010;31:607–617. - PubMed
-
- Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135:173–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

