Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: immunophenotypic characterization and differentiation potential
- PMID: 24559797
- PMCID: PMC4055040
- DOI: 10.1186/scrt414
Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: immunophenotypic characterization and differentiation potential
Abstract
Introduction: Studies with mesenchymal stem cells (MSCs) are increasing due to their immunomodulatory, anti-inflammatory and tissue regenerative properties. However, there is still no agreement about the best source of equine MSCs for a bank for allogeneic therapy. The aim of this study was to evaluate the cell culture and immunophenotypic characteristics and differentiation potential of equine MSCs from bone marrow (BM-MSCs), adipose tissue (AT-MSCs) and umbilical cord (UC-MSCs) under identical in vitro conditions, to compare these sources for research or an allogeneic therapy cell bank.
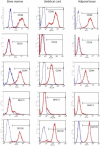
Methods: The BM-MSCs, AT-MSCs and UC-MSCs were cultured and evaluated in vitro for their osteogenic, adipogenic and chondrogenic differentiation potential. Additionally, MSCs were assessed for CD105, CD44, CD34, CD90 and MHC-II markers by flow cytometry, and MHC-II was also assessed by immunocytochemistry. To interpret the flow cytometry results, statistical analysis was performed using ANOVA.
Results: The harvesting and culturing procedures of BM-MSCs, AT-MSCs and UC-MSCs were feasible, with an average cell growth until the third passage of 25 days for BM-MSCs, 15 days for AT-MSCs and 26 days for UC-MSCs. MSCs from all sources were able to differentiate into osteogenic (after 10 days for BM-MSCs and AT-MSCs and 15 days for UC-MSCs), adipogenic (after 8 days for BM-MSCs and AT-MSCs and 15 days for UC-MSCs) and chondrogenic (after 21 days for BM-MSCs, AT-MSCs and UC-MSCs) lineages. MSCs showed high expression of CD105, CD44 and CD90 and low or negative expression of CD34 and MHC-II. The MHC-II was not detected by immunocytochemistry techniques in any of the MSCs studied.
Conclusions: The BM, AT and UC are feasible sources for harvesting equine MSCs, and their immunophenotypic and multipotency characteristics attained minimal criteria for defining MSCs. Due to the low expression of MHC-II by MSCs, all of the sources could be used in clinical trials involving allogeneic therapy in horses. However, the BM-MSCs and AT-MSCs showed fastest ''in vitro'' differentiation and AT-MSCs showed highest cell growth until third passage. These findings suggest that BM and AT may be preferable for cell banking purposes.
Figures



Similar articles
-
Biological characteristics and metabolic profile of canine mesenchymal stem cells isolated from adipose tissue and umbilical cord matrix.PLoS One. 2021 Mar 4;16(3):e0247567. doi: 10.1371/journal.pone.0247567. eCollection 2021. PLoS One. 2021. PMID: 33661930 Free PMC article.
-
Blood type and breed-associated differences in cell marker expression on equine bone marrow-derived mesenchymal stem cells including major histocompatibility complex class II antigen expression.PLoS One. 2019 Nov 20;14(11):e0225161. doi: 10.1371/journal.pone.0225161. eCollection 2019. PLoS One. 2019. PMID: 31747418 Free PMC article.
-
Adipogenic potentials of mesenchymal stem cells from human bone marrow, umbilical cord and adipose tissue are different.Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2014 Jun;22(3):588-94. doi: 10.7534/j.issn.1009-2137.2014.03.003. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2014. PMID: 24989259
-
The biology of equine mesenchymal stem cells: phenotypic characterization, cell surface markers and multilineage differentiation.Front Biosci (Landmark Ed). 2012 Jan 1;17(3):892-908. doi: 10.2741/3963. Front Biosci (Landmark Ed). 2012. PMID: 22201780 Review.
-
Mesenchymal stem cells in the umbilical cord: phenotypic characterization, secretome and applications in central nervous system regenerative medicine.Curr Stem Cell Res Ther. 2011 Sep;6(3):221-8. doi: 10.2174/157488811796575332. Curr Stem Cell Res Ther. 2011. PMID: 21476975 Review.
Cited by
-
Therapeutic Use of Mesenchymal Stromal Cells: The Need for Inclusive Characterization Guidelines to Accommodate All Tissue Sources and Species.Front Cell Dev Biol. 2021 Feb 16;9:632717. doi: 10.3389/fcell.2021.632717. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33665190 Free PMC article. Review.
-
Applications of mesenchymal stem cell technology in bovine species.Stem Cell Res Ther. 2019 Jan 24;10(1):44. doi: 10.1186/s13287-019-1145-9. Stem Cell Res Ther. 2019. PMID: 30678726 Free PMC article. Review.
-
Development of a biologically immortalized equine stem cell line.Can J Vet Res. 2021 Oct;85(4):293-301. Can J Vet Res. 2021. PMID: 34602734 Free PMC article.
-
Mesenchymal Stem Cell-Derived Exosomes: Biological Function and Their Therapeutic Potential in Radiation Damage.Cells. 2020 Dec 30;10(1):42. doi: 10.3390/cells10010042. Cells. 2020. PMID: 33396665 Free PMC article. Review.
-
Osteogenic Differentiation of Adipose Tissue-Derived Mesenchymal Stem Cells on Composite Polymeric Scaffolds: A Review.Curr Stem Cell Res Ther. 2025;20(1):33-49. doi: 10.2174/011574888X263333231218065453. Curr Stem Cell Res Ther. 2025. PMID: 38315659 Review.
References
-
- Carrade DD, Owens SD, Galuppo LD, Vidal MA, Ferraro GL, Librach F, Buerchler S, Friedman MS, Walker NJ, Borjesson DL. Clinicopathologic findings following intra-articular injection of autologous and allogeneic placentally derived equine mesenchymal stem cells in horses. Cytotherapy. 2011;13:419–430. doi: 10.3109/14653249.2010.536213. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

