Self crowding of globular proteins studied by small-angle x-ray scattering
- PMID: 24559992
- PMCID: PMC3944889
- DOI: 10.1016/j.bpj.2013.12.004
Self crowding of globular proteins studied by small-angle x-ray scattering
Abstract
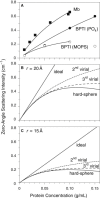
Small-angle x-ray scattering (SAXS) was used to study the behavior of equine metmyoglobin (Mb) and bovine pancreatic trypsin inhibitor (BPTI) at concentrations up to 0.4 and 0.15 g/mL, respectively, in solutions also containing 50% D2O and 1 M urea. For both proteins, significant effects because of interference between x-rays scattered by different molecules (interparticle interference) were observed, indicating nonideal behavior at high concentrations. The experimental data were analyzed by comparison of the observed scattering profiles with those predicted by crystal structures of the proteins and a hard-sphere fluid model used to represent steric exclusion effects. The Mb scattering data were well fit by the hard-sphere model using a sphere radius of 18 Å, only slightly smaller than that estimated from the three-dimensional structure (20 Å). In contrast, the scattering profiles for BPTI in phosphate buffer displayed substantially less pronounced interparticle interference than predicted by the hard-sphere model and the radius estimated from the known structure of the protein (15 Å). Replacing the phosphate buffer with 3-(N-morpolino)propane sulfonic acid (MOPS) led to increased interparticle interference, consistent with a larger effective radius and suggesting that phosphate ions may mediate attractive intermolecular interactions, as observed in some BPTI crystal structures, without the formation of stable oligomers. The scattering data were also used to estimate second virial coefficients for the two proteins: 2.0 ×10(-4) cm(3)mol/g(2) for Mb in phosphate buffer, 1.6 ×10(-4) cm(3)mol/g(2) for BPTI in phosphate buffer and 9.2 ×10(-4) cm(3)mol/g(2) for BPTI in MOPS. The results indicate that the behavior of Mb, which is nearly isoelectric under the conditions used, is well described by the hard-sphere model, but that of BPTI is considerably more complex and is likely influenced by both repulsive and attractive electrostatic interactions. The hard-sphere model may be a generally useful tool for the analysis of small-angle scattering data from concentrated macromolecular solutions.
Copyright © 2014 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures





Comment in
-
SAXS/SANS probe of intermolecular interactions in concentrated protein solutions.Biophys J. 2014 Feb 18;106(4):771-3. doi: 10.1016/j.bpj.2014.01.019. Biophys J. 2014. PMID: 24559977 Free PMC article. No abstract available.
References
-
- Saluja A., Kalonia D.S. Nature and consequences of protein-protein interactions in high protein concentration solutions. Int. J. Pharm. 2008;358:1–15. - PubMed
-
- Shire S.J. Formulation and manufacturability of biologics. Curr. Opin. Biotechnol. 2009;20:708–714. - PubMed
-
- Wiencek J.M. New strategies for protein crystal growth. Annu. Rev. Biomed. Eng. 1999;1:505–534. - PubMed
-
- George A., Wilson W.W. Predicting protein crystallization from a dilute solution property. Acta Crystallogr. D Biol. Crystallogr. 1994;50:361–365. - PubMed
-
- Bonneté F., Finet S., Tardieu A. Second virial coefficient: variations with lysozyme crystallization conditions. J. Cryst. Growth. 1999;196:403–414.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

