Comparison between the behavior of different hydrophobic peptides allowing membrane anchoring of proteins
- PMID: 24560216
- PMCID: PMC4028306
- DOI: 10.1016/j.cis.2014.01.015
Comparison between the behavior of different hydrophobic peptides allowing membrane anchoring of proteins
Abstract
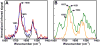
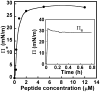
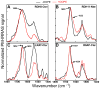
Membrane binding of proteins such as short chain dehydrogenase reductases or tail-anchored proteins relies on their N- and/or C-terminal hydrophobic transmembrane segment. In this review, we propose guidelines to characterize such hydrophobic peptide segments using spectroscopic and biophysical measurements. The secondary structure content of the C-terminal peptides of retinol dehydrogenase 8, RGS9-1 anchor protein, lecithin retinol acyl transferase, and of the N-terminal peptide of retinol dehydrogenase 11 has been deduced by prediction tools from their primary sequence as well as by using infrared or circular dichroism analyses. Depending on the solvent and the solubilization method, significant structural differences were observed, often involving α-helices. The helical structure of these peptides was found to be consistent with their presumed membrane binding. Langmuir monolayers have been used as membrane models to study lipid-peptide interactions. The values of maximum insertion pressure obtained for all peptides using a monolayer of 1,2-dioleoyl-sn-glycero-3-phospho-ethanolamine (DOPE) are larger than the estimated lateral pressure of membranes, thus suggesting that they bind membranes. Polarization modulation infrared reflection absorption spectroscopy has been used to determine the structure and orientation of these peptides in the absence and in the presence of a DOPE monolayer. This lipid induced an increase or a decrease in the organization of the peptide secondary structure. Further measurements are necessary using other lipids to better understand the membrane interactions of these peptides.
Keywords: Circular dichroism and infrared spectroscopy; Lecithin retinol acyltransferase; Monolayer; R9AP; Retinol dehydrogenase; Transmembrane hydrophobic peptide.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures







References
-
- Yeagle PL, Albert AD. Biochim Biophys Acta. 2007;1768:808. - PubMed
-
- Chini B, Parenti M, Poyner DR, Wheatley M. Biochem Soc Trans. 2013;41:135. - PubMed
-
- Audet M, Bouvier M. Cell. 2012;151:14. - PubMed
-
- Hofmann KP, Scheerer P, Hildebrand PW, Choe HW, Park JH, Heck M, et al. Trends Biochem Sci. 2009;34:540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

