Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis
- PMID: 24560412
- PMCID: PMC4007098
- DOI: 10.1016/j.jaci.2014.01.021
Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis
Abstract
Background: Exposure to food allergens through a disrupted skin barrier has been recognized as a potential factor in the increasing prevalence of food allergy.
Objective: We sought to test the immunologic mechanisms by which epicutaneous sensitization to food allergens predisposes to intestinal food allergy.
Methods: Mice were epicutaneously sensitized with ovalbumin or peanut on an atopic dermatitis-like skin lesion, followed by intragastric antigen challenge. Antigen-specific serum IgE levels and T(H)2 cytokine responses were measured by ELISA. Expression of type 2 cytokines and mast cell proteases in the intestine were measured by using real-time PCR. Accumulation of basophils in the skin and mast cells in the intestine was examined by using flow cytometry. In vivo basophil depletion was achieved by using diphtheria toxin treatment of Baso-DTR mice. For cell-transfer studies, the basophil population was expanded in vivo by means of hydrodynamic tail vein injection of thymic stromal lymphopoietin (TSLP) cDNA plasmid.
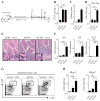
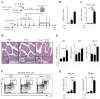
Results: Sensitization to food allergens through an atopic dermatitis-like skin lesion is associated with an expansion of TSLP-elicited basophils in the skin that promote antigen-specific T(H)2 cytokine responses, increased antigen-specific serum IgE levels, and accumulation of mast cells in the intestine, promoting the development of intestinal food allergy. Critically, disruption of TSLP responses or depletion of basophils reduced the susceptibility to intestinal food allergy, whereas transfer of TSLP-elicited basophils into intact skin promoted disease.
Conclusion: Epicutaneous sensitization on a disrupted skin barrier is associated with accumulation of TSLP-elicited basophils, which are necessary and sufficient to promote antigen-induced intestinal food allergy.
Keywords: Food allergy; IgE; atopic dermatitis; basophils; epicutaneous sensitization; mast cells; thymic stromal lymphopoietin.
Copyright © 2014 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: M.R.C is an employee and shareholder of Amgen.
Figures




References
-
- Sicherer SH, Sampson HA. Food allergy. The Journal of allergy and clinical immunology. 2010;125(2 Suppl 2):S116–25. - PubMed
-
- Burks AW, Tang M, Sicherer S, Muraro A, Eigenmann PA, Ebisawa M, et al. ICON: food allergy. The Journal of allergy and clinical immunology. 2012;129(4):906–20. - PubMed
-
- Fiocchi A, Schunemann HJ, Brozek J, Restani P, Beyer K, Troncone R, et al. Diagnosis and Rationale for Action Against Cow’s Milk Allergy (DRACMA): a summary report. The Journal of allergy and clinical immunology. 2010;126(6):1119–28. e12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI106679/AI/NIAID NIH HHS/United States
- R01 AI095466/AI/NIAID NIH HHS/United States
- AI061570/AI/NIAID NIH HHS/United States
- P30 DK050306/DK/NIDDK NIH HHS/United States
- F32 AI085828/AI/NIAID NIH HHS/United States
- AI074878/AI/NIAID NIH HHS/United States
- AI087990/AI/NIAID NIH HHS/United States
- R01 AI102942/AI/NIAID NIH HHS/United States
- R21 AI083480/AI/NIAID NIH HHS/United States
- U01 AI095608/AI/NIAID NIH HHS/United States
- R01 AI061570/AI/NIAID NIH HHS/United States
- R01 AI074878/AI/NIAID NIH HHS/United States
- K08 AR065577/AR/NIAMS NIH HHS/United States
- P01 AI106697/AI/NIAID NIH HHS/United States
- 2-P30- CA016520/CA/NCI NIH HHS/United States
- KL2-RR024132/RR/NCRR NIH HHS/United States
- AI102942/AI/NIAID NIH HHS/United States
- P30 AR057217/AR/NIAMS NIH HHS/United States
- R21 AI087990/AI/NIAID NIH HHS/United States
- KL2 RR024132/RR/NCRR NIH HHS/United States
- T32 AR007465/AR/NIAMS NIH HHS/United States
- AI095608/AI/NIAID NIH HHS/United States
- P30 CA016520/CA/NCI NIH HHS/United States
- P30-DK050306/DK/NIDDK NIH HHS/United States
- T32-AR007465/AR/NIAMS NIH HHS/United States
- R01 AI097333/AI/NIAID NIH HHS/United States
- AI095466/AI/NIAID NIH HHS/United States
- F32-AI085828/AI/NIAID NIH HHS/United States
- AI097333/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

