Evoked and spontaneous transmission favored by distinct sets of synapses
- PMID: 24560571
- PMCID: PMC4017949
- DOI: 10.1016/j.cub.2014.01.022
Evoked and spontaneous transmission favored by distinct sets of synapses
Abstract
Background: Spontaneous "miniature" transmitter release takes place at low rates at all synapses. Long thought of as an unavoidable leak, spontaneous release has recently been suggested to be mediated by distinct pre- and postsynaptic molecular machineries and to have a specialized role in setting up and adjusting neuronal circuits. It remains unclear how spontaneous and evoked transmission are related at individual synapses, how they are distributed spatially when an axon makes multiple contacts with a target, and whether they are commonly regulated.
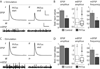
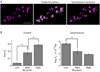
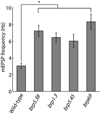
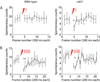
Results: Electrophysiological recordings in the Drosophila larval neuromuscular junction, in the presence of the use-dependent glutamate receptor (GluR) blocker philanthotoxin, indicated that spontaneous and evoked transmission employ distinct sets of GluRs. In vivo imaging of transmission using synaptically targeted GCaMP3 to detect Ca(2+) influx through the GluRs revealed little spatial overlap between synapses participating in spontaneous and evoked transmission. Spontaneous and evoked transmission were oppositely correlated with presynaptic levels of the protein Brp: synapses with high Brp favored evoked transmission, whereas synapses with low Brp were more active spontaneously. High-frequency stimulation did not increase the overlap between evoked and spontaneous transmission, and instead decreased the rate of spontaneous release from synapses that were highly active in evoked transmission.
Conclusions: Although individual synapses can participate in both evoked and spontaneous transmission, highly active synapses show a preference for one mode of transmission. The presynaptic protein Brp promotes evoked transmission and suppresses spontaneous release. These findings suggest the existence of presynaptic mechanisms that promote synaptic specialization to either evoked or spontaneous transmission.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures



Comment in
-
Neurotransmission: spontaneous and evoked release filing for divorce.Curr Biol. 2014 Mar 3;24(5):R192-4. doi: 10.1016/j.cub.2014.01.037. Curr Biol. 2014. PMID: 24602882
Similar articles
-
Structural Remodeling of Active Zones Is Associated with Synaptic Homeostasis.J Neurosci. 2020 Apr 1;40(14):2817-2827. doi: 10.1523/JNEUROSCI.2002-19.2020. Epub 2020 Mar 2. J Neurosci. 2020. PMID: 32122953 Free PMC article.
-
RIM promotes calcium channel accumulation at active zones of the Drosophila neuromuscular junction.J Neurosci. 2012 Nov 21;32(47):16586-96. doi: 10.1523/JNEUROSCI.0965-12.2012. J Neurosci. 2012. PMID: 23175814 Free PMC article.
-
Synaptic excitation is regulated by the postsynaptic dSK channel at the Drosophila larval NMJ.J Neurophysiol. 2014 Jun 15;111(12):2533-43. doi: 10.1152/jn.00903.2013. Epub 2014 Mar 26. J Neurophysiol. 2014. PMID: 24671529 Free PMC article.
-
Glutamate receptors in synaptic assembly and plasticity: case studies on fly NMJs.Adv Exp Med Biol. 2012;970:3-28. doi: 10.1007/978-3-7091-0932-8_1. Adv Exp Med Biol. 2012. PMID: 22351049 Review.
-
Receptor clustering: nothing succeeds like success.Curr Biol. 2004 Jun 8;14(11):R413-5. doi: 10.1016/j.cub.2004.05.031. Curr Biol. 2004. PMID: 15182686 Review.
Cited by
-
(M)Unc13s in Active Zone Diversity: A Drosophila Perspective.Front Synaptic Neurosci. 2022 Jan 3;13:798204. doi: 10.3389/fnsyn.2021.798204. eCollection 2021. Front Synaptic Neurosci. 2022. PMID: 35046788 Free PMC article. Review.
-
Distinct molecular pathways govern presynaptic homeostatic plasticity.Cell Rep. 2021 Dec 14;37(11):110105. doi: 10.1016/j.celrep.2021.110105. Cell Rep. 2021. PMID: 34910905 Free PMC article.
-
Stochastic Properties of Spontaneous Synaptic Transmission at Individual Active Zones.J Neurosci. 2022 Feb 9;42(6):1001-1019. doi: 10.1523/JNEUROSCI.1162-21.2021. Epub 2021 Dec 30. J Neurosci. 2022. PMID: 34969867 Free PMC article.
-
Targeting Homeostatic Synaptic Plasticity for Treatment of Mood Disorders.Neuron. 2020 Jun 3;106(5):715-726. doi: 10.1016/j.neuron.2020.05.015. Neuron. 2020. PMID: 32497508 Free PMC article. Review.
-
Structural Remodeling of Active Zones Is Associated with Synaptic Homeostasis.J Neurosci. 2020 Apr 1;40(14):2817-2827. doi: 10.1523/JNEUROSCI.2002-19.2020. Epub 2020 Mar 2. J Neurosci. 2020. PMID: 32122953 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

