Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury
- PMID: 24560714
- PMCID: PMC4036591
- DOI: 10.1016/j.expneurol.2014.02.013
Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury
Abstract
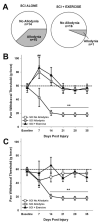
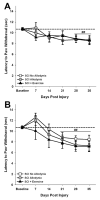
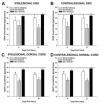
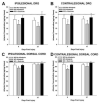
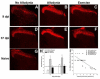
Spinal cord injury (SCI) impaired sensory fiber transmission leads to chronic, debilitating neuropathic pain. Sensory afferents are responsive to neurotrophic factors, molecules that are known to promote survival and maintenance of neurons, and regulate sensory neuron transduction of peripheral stimuli. A subset of primary afferent fibers responds only to the glial cell-line derived neurotrophic factor (GDNF) family of ligands (GFLs) and is non-peptidergic. In peripheral nerve injury models, restoration of GDNF or artemin (another GFL) to pre-injury levels within the spinal cord attenuates neuropathic pain. One non-invasive approach to increase the levels of GFLs in the spinal cord is through exercise (Ex), and to date exercise training is the only ameliorative, non-pharmacological treatment for SCI-induced neuropathic pain. The purpose of this study was 3-fold: 1) to determine whether exercise affects the onset of SCI-induced neuropathic pain; 2) to examine the temporal profile of GDNF and artemin in the dorsal root ganglia and spinal cord dorsal horn regions associated with forepaw dermatomes after SCI and Ex; and 3) to characterize GFL-responsive sensory fiber plasticity after SCI and Ex. Adult, female, Sprague-Dawley rats received a moderate, unilateral spinal cord contusion at C5. A subset of rats was exercised (SCI+Ex) on automated running wheels for 20min, 5days/week starting at 5days post-injury (dpi), continuing until 9 or 37dpi. Hargreaves' and von Frey testing was performed preoperatively and weekly post-SCI. Forty-two percent of rats in the unexercised group exhibited tactile allodynia of the forepaws while the other 58% retained normal sensation. The development of SCI-induced neuropathic pain correlated with a marked decrease in the levels of GDNF and artemin in the spinal cord and DRGs. Additionally, a dramatic increase in the density and the distribution throughout the dorsal horn of GFL-responsive afferents was observed in rats with SCI-induced allodynia. Importantly, in SCI rats that received Ex, the incidence of tactile allodynia decreased to 7% (1/17) and there was maintenance of GDNF and artemin at normal levels, with a normal distribution of GFL-responsive fibers. These data suggest that GFLs and/or their downstream effectors may be important modulators of pain fiber plasticity, representing effective targets for anti-allodynic therapeutics. Furthermore, we highlight the potent beneficial effects of acute exercise after SCI.
Keywords: Artemin; Central pain; GDNF; Mechanical allodynia; Spinal cord injury; Thermal hyperalgesia.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Delayed Exercise Is Ineffective at Reversing Aberrant Nociceptive Afferent Plasticity or Neuropathic Pain After Spinal Cord Injury in Rats.Neurorehabil Neural Repair. 2016 Aug;30(7):685-700. doi: 10.1177/1545968315619698. Epub 2015 Dec 14. Neurorehabil Neural Repair. 2016. PMID: 26671215 Free PMC article.
-
Persistent at-level thermal hyperalgesia and tactile allodynia accompany chronic neuronal and astrocyte activation in superficial dorsal horn following mouse cervical contusion spinal cord injury.PLoS One. 2014 Sep 30;9(9):e109099. doi: 10.1371/journal.pone.0109099. eCollection 2014. PLoS One. 2014. PMID: 25268642 Free PMC article.
-
Upregulation of calcium channel alpha-2-delta-1 subunit in dorsal horn contributes to spinal cord injury-induced tactile allodynia.Spine J. 2018 Jun;18(6):1062-1069. doi: 10.1016/j.spinee.2018.01.010. Epub 2018 Jan 31. Spine J. 2018. PMID: 29355786
-
Pain with no gain: allodynia following neural stem cell transplantation in spinal cord injury.Exp Neurol. 2006 Oct;201(2):335-48. doi: 10.1016/j.expneurol.2006.04.035. Epub 2006 Jul 12. Exp Neurol. 2006. PMID: 16839548
-
Chronic at- and below-level pain after moderate unilateral cervical spinal cord contusion in rats.J Neurotrauma. 2013 May 15;30(10):884-90. doi: 10.1089/neu.2012.2632. J Neurotrauma. 2013. PMID: 23216008 Free PMC article.
Cited by
-
A review of dorsal root ganglia and primary sensory neuron plasticity mediating inflammatory and chronic neuropathic pain.Neurobiol Pain. 2024 Jan 20;15:100151. doi: 10.1016/j.ynpai.2024.100151. eCollection 2024 Jan-Jun. Neurobiol Pain. 2024. PMID: 38314104 Free PMC article. Review.
-
Emerging Relationships between Exercise, Sensory Nerves, and Neuropathic Pain.Front Neurosci. 2016 Aug 23;10:372. doi: 10.3389/fnins.2016.00372. eCollection 2016. Front Neurosci. 2016. PMID: 27601974 Free PMC article. Review.
-
Phylogenetic view of the compensatory mechanisms in motor and sensory systems after neuronal injury.Curr Res Neurobiol. 2022 Oct 17;3:100058. doi: 10.1016/j.crneur.2022.100058. eCollection 2022. Curr Res Neurobiol. 2022. PMID: 36304591 Free PMC article. Review.
-
C-low threshold mechanoreceptor activation becomes sufficient to trigger affective pain in spinal cord-injured mice in association with increased respiratory rates.Front Integr Neurosci. 2022 Dec 21;16:1081172. doi: 10.3389/fnint.2022.1081172. eCollection 2022. Front Integr Neurosci. 2022. PMID: 36619238 Free PMC article.
-
Exercise training modulates glutamic acid decarboxylase-65/67 expression through TrkB signaling to ameliorate neuropathic pain in rats with spinal cord injury.Mol Pain. 2020 Jan-Dec;16:1744806920924511. doi: 10.1177/1744806920924511. Mol Pain. 2020. PMID: 32418502 Free PMC article.
References
-
- Boucher TJ, McMahon SB. Neurotrophic factors and neuropathic pain. Curr Opin Pharmacol. 2001;1:66–72. - PubMed
-
- Boucher TJ, Okuse K, Bennett DL, Munson JB, Wood JN, McMahon SB. Potent analgesic effects of GDNF in neuropathic pain states. Science. 2000;290:124–7. - PubMed
-
- Calancie B, Molano MR, Broton JG. Epidemiology and demography of acute spinal cord injury in a large urban setting. J Spinal Cord Med. 2005;28:92–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

