PTEN C-terminal deletion causes genomic instability and tumor development
- PMID: 24561254
- PMCID: PMC4090077
- DOI: 10.1016/j.celrep.2014.01.030
PTEN C-terminal deletion causes genomic instability and tumor development
Abstract
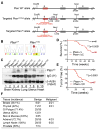
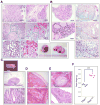
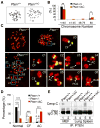
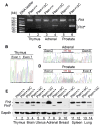
Tumor suppressor PTEN controls genomic stability and inhibits tumorigenesis. The N-terminal phosphatase domain of PTEN antagonizes the PI3K/AKT pathway, but its C-terminal function is less defined. Here, we describe a knockin mouse model of a nonsense mutation that results in the deletion of the entire Pten C-terminal region, referred to as Pten(ΔC). Mice heterozygous for Pten(ΔC) develop multiple spontaneous tumors, including cancers and B cell lymphoma. Heterozygous deletion of the Pten C-terminal domain also causes genomic instability and common fragile site rearrangement. We found that Pten C-terminal disruption induces p53 and its downstream targets. Simultaneous depletion of p53 promotes metastasis without influencing the initiation of tumors, suggesting that p53 mainly suppresses tumor progression. Our data highlight the essential role of the PTEN C terminus in the maintenance of genomic stability and suppression of tumorigenesis.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Anzelon AN, Wu H, Rickert RC. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat Immunol. 2003;4:287–294. - PubMed
-
- Basso K, Dalla-Favera R. BCL6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv Immunol. 2010;105:193–210. - PubMed
-
- Bubien V, Bonnet F, Brouste V, Hoppe S, Barouk-Simonet E, David A, Edery P, Bottani A, Layet V, Caron O, et al. High cumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. J Med Genet. 2013;50:255–263. - PubMed
-
- Cattoretti G, Pasqualucci L, Ballon G, Tam W, Nandula SV, Shen Q, Mo T, Murty VV, Dalla-Favera R. Deregulated BCL6 expression recapitulates the pathogenesis of human diffuse large B cell lymphomas in mice. Cancer Cell. 2005;7:445–455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

