Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes β cell regeneration
- PMID: 24561261
- PMCID: PMC4012856
- DOI: 10.1016/j.cmet.2014.02.001
Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes β cell regeneration
Erratum in
- Cell Metab. 2015 Oct 6;22(4):750
Abstract
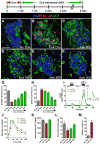
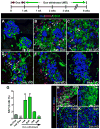
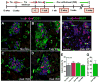
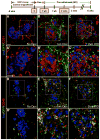
Pancreatic islet endocrine cell and endothelial cell (EC) interactions mediated by vascular endothelial growth factor-A (VEGF-A) signaling are important for islet differentiation and the formation of highly vascularized islets. To dissect how VEGF-A signaling modulates intra-islet vasculature, islet microenvironment, and β cell mass, we transiently increased VEGF-A production by β cells. VEGF-A induction dramatically increased the number of intra-islet ECs but led to β cell loss. After withdrawal of the VEGF-A stimulus, β cell mass, function, and islet structure normalized as a result of a robust, but transient, burst in proliferation of pre-existing β cells. Bone marrow-derived macrophages (MΦs) recruited to the site of β cell injury were crucial for the β cell proliferation, which was independent of pancreatic location and circulating factors such as glucose. Identification of the signals responsible for the proliferation of adult, terminally differentiated β cells will improve strategies aimed at β cell regeneration and expansion.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



References
-
- Banaei-Bouchareb L, Gouon-Evans V, Samara-Boustani D, Castellotti MC, Czernichow P, Pollard JW, Polak M. Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. J Leukoc Biol. 2004;76:359–367. - PubMed
-
- Bardin N, Blot-Chabaud M, Despoix N, Kebir A, Harhouri K, Arsanto JP, Espinosa L, Perrin P, Robert S, Vely F, et al. CD146 and its soluble form regulate monocyte transendothelial migration. Arterioscler Thromb Vasc Biol. 2009;29:746–753. - PubMed
-
- Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marmé D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P60 DK020593/DK/NIDDK NIH HHS/United States
- DK69603/DK/NIDDK NIH HHS/United States
- DK58404/DK/NIDDK NIH HHS/United States
- P30 CA68485/CA/NCI NIH HHS/United States
- F30 DK097921/DK/NIDDK NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- U01 DK072473/DK/NIDDK NIH HHS/United States
- EY08126/EY/NEI NIH HHS/United States
- U01 DK089572/DK/NIDDK NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- R33 DK066636/DK/NIDDK NIH HHS/United States
- R01 DK069603/DK/NIDDK NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- R01 DK068764/DK/NIDDK NIH HHS/United States
- HD15052/HD/NICHD NIH HHS/United States
- DK058404/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- R21 DK062641/DK/NIDDK NIH HHS/United States
- DK089572/DK/NIDDK NIH HHS/United States
- DK09538/DK/NIDDK NIH HHS/United States
- DK68764/DK/NIDDK NIH HHS/United States
- DK66636/DK/NIDDK NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- DK59637/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- DK72473/DK/NIDDK NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- DK63439/DK/NIDDK NIH HHS/United States
- U01 DK089538/DK/NIDDK NIH HHS/United States
- DK62641/DK/NIDDK NIH HHS/United States
- R21 DK066636/DK/NIDDK NIH HHS/United States
- R21 DK063439/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

