KCNJ2 mutation causes an adrenergic-dependent rectification abnormality with calcium sensitivity and ventricular arrhythmia
- PMID: 24561538
- PMCID: PMC4257478
- DOI: 10.1016/j.hrthm.2014.02.015
KCNJ2 mutation causes an adrenergic-dependent rectification abnormality with calcium sensitivity and ventricular arrhythmia
Abstract
Background: KCNJ2 mutations are associated with a variety of inherited arrhythmia syndromes including catecholaminergic polymorphic ventricular tachycardia 3.
Objective: To characterize the detailed cellular mechanisms of the clinically recognized KCNJ2 mutation R67Q.
Methods: Kir2.1 current density was measured from COS-1 cells transiently transfected with wild-type human Kir-2.1 (WT-Kir2.1) and/or a heterozygous missense mutation in KCNJ2 (R67Q-Kir2.1) by using the whole-cell voltage clamp technique. Catecholamine activity was simulated with protein kinase A-stimulating cocktail exposure. Phosphorylation-deficient mutants, S425N-Kir2.1 and S425N-Kir2.1/R67Q-S425N-Kir2.1, were used in a separate set of experiments. HA- or Myc-Tag-WT-Kir2.1 and HA-Tag-R67Q-Kir2.1 were used for confocal imaging.
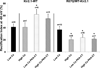
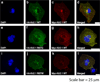
Results: A 33-year-old woman presented with a catecholaminergic polymorphic ventricular tachycardia-like clinical phenotype and was found to have KCNJ2 missense mutation R67Q. Treatment with nadolol and flecainide resulted in the complete suppression of arrhythmias and symptom resolution. Under baseline conditions, R67Q-Kir2.1 expressed alone did not produce inward rectifier current while cells coexpressing WT-Kir2.1 and R67Q-Kir2.1 demonstrated the rectification index (RI) similar to that of WT-Kir2.1. After PKA stimulation, R67Q-Kir2.1/WT-Kir2.1 failed to increase peak outward current density; WT-Kir2.1 increased by 46% (n = 5), while R67Q-Kir2.1/WT-Kir2.1 decreased by 6% (n = 6) (P = .002). Rectification properties in R67Q-Kir2.1/WT-Kir2.1 demonstrated sensitivity to calcium with a decreased RI in the high-calcium pipette solution (RI 20.3% ± 4.1%) than in the low-calcium pipette solution (RI 36.5% ± 5.7%) (P < .05). Immunostaining of WT-Kir2.1 and R67Q-Kir2.1 individually and together showed a normal membrane expression pattern and colocalization by using the Pearson correlation coefficient.
Conclusions: R67Q-Kir2.1 is associated with an adrenergic-dependent clinical and cellular phenotype with rectification abnormality enhanced by increased calcium. These findings are a significant advancement of our knowledge and understanding of the phenotype-genotype relationship of arrhythmia syndromes related to KCNJ2 mutations.
Keywords: Arrhythmia syndrome; CPVT3; Inherited arrhythmia; KCNJ2; Kir2.1; Potassium inward-rectifier channel; Ventricular arrhythmia.
Copyright © 2014 Heart Rhythm Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors report no conflicts of interest.
Figures





References
-
- Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc D, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children: a 7-year follow up of 21 patients. Circulation. 1995;91:1512–1519. - PubMed
-
- Priori SG, Napolitano C, Tiso N, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. - PubMed
-
- Kontula K, Laitinen PJ, Lehtonen A, Toivonen L, Viitasalo M, Swan H. Catecholaminergic polymorphic ventricular tachycardia: recent mechanistic insights. Cardiovascular research. 2005;67:379–387. - PubMed
-
- Tester D, Arya P, Will M, et al. Genotypic heterogeneity and phenotypic mimicry among unrelated patients referred for catecholaminergic polymorphic ventricular tachycardia genetic testing. Heart rhythm : the official journal of the Heart Rhythm Society. 2006;3:800–805. - PubMed
-
- Chun TU, Epstein MR, Dick M, 2nd, et al. Polymorphic ventricular tachycardia and KCNJ2 mutations. Heart rhythm : the official journal of the Heart Rhythm Society. 2004;1:235–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

