Dimethylglycine provides salt and temperature stress protection to Bacillus subtilis
- PMID: 24561588
- PMCID: PMC3993278
- DOI: 10.1128/AEM.00078-14
Dimethylglycine provides salt and temperature stress protection to Bacillus subtilis
Abstract
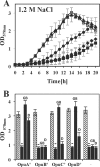
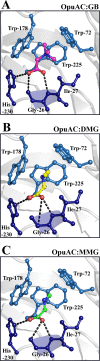
Glycine betaine is a potent osmotic and thermal stress protectant of many microorganisms. Its synthesis from glycine results in the formation of the intermediates monomethylglycine (sarcosine) and dimethylglycine (DMG), and these compounds are also produced when it is catabolized. Bacillus subtilis does not produce sarcosine or DMG, and it cannot metabolize these compounds. Here we have studied the potential of sarcosine and DMG to protect B. subtilis against osmotic, heat, and cold stress. Sarcosine, a compatible solute that possesses considerable protein-stabilizing properties, did not serve as a stress protectant of B. subtilis. DMG, on the other hand, proved to be only moderately effective as an osmotic stress protectant, but it exhibited good heat stress-relieving and excellent cold stress-relieving properties. DMG is imported into B. subtilis cells primarily under osmotic and temperature stress conditions via OpuA, a member of the ABC family of transporters. Ligand-binding studies with the extracellular solute receptor (OpuAC) of the OpuA system showed that OpuAC possesses a moderate affinity for DMG, with a Kd value of approximate 172 μM; its Kd for glycine betaine is about 26 μM. Docking studies using the crystal structures of the OpuAC protein with the sulfur analog of DMG, dimethylsulfonioacetate, as a template suggest a model of how the DMG molecule can be stably accommodated within the aromatic cage of the OpuAC ligand-binding pocket. Collectively, our data show that the ability to acquire DMG from exogenous sources under stressful environmental conditions helps the B. subtilis cell to cope with growth-restricting osmotic and temperature challenges.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

