Interactions between the non-seed region of siRNA and RNA-binding RLC/RISC proteins, Ago and TRBP, in mammalian cells
- PMID: 24561616
- PMCID: PMC4005638
- DOI: 10.1093/nar/gku153
Interactions between the non-seed region of siRNA and RNA-binding RLC/RISC proteins, Ago and TRBP, in mammalian cells
Abstract
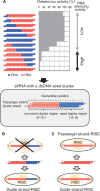
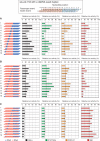
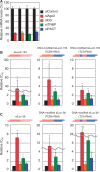
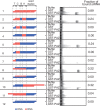
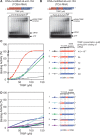
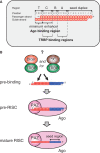
Small interfering RNA (siRNA)-based RNA interference (RNAi) is widely used for target gene silencing in various organisms. We previously showed that 8-nt-long 5' proximal nucleotides, which include seed sequence (positions 2-8 from the 5' end of guide strand), and the complementary sequence of the passenger strand are capable of being simultaneously replaced with cognate deoxyribonucleotides without any substantial loss of gene silencing. In the present study, examination was made of RNA requirements in the non-seed region of siRNA. The non-seed region of siRNA was found to be subdivided into four domains, in which two nucleotide pairs (positions 13 and 14) were replaceable with cognate deoxyribonucleotides without reducing RNAi activity. However, RNA sequences at positions 9-12 and 15-18 were essential for effective gene silencing, and these two double-stranded RNA cores are required for binding of the trans-activation response RNA-binding protein (TRBP). The terminal RNA (positions 19-21) provided Argonaute protein binding sites. Argonaute binding was enhanced by the presence of RNAs at positions 15-18. Knockdown experiments showed that, unlike Argonaute and TRBP, Dicer was dispensable for RNAi. Based on these observations, we discuss possible RNA/protein and protein/protein interactions in RNA-induced silencing complex formation.
Figures

Similar articles
-
Multiple sensors ensure guide strand selection in human RNAi pathways.RNA. 2013 May;19(5):639-48. doi: 10.1261/rna.037424.112. Epub 2013 Mar 26. RNA. 2013. PMID: 23531496 Free PMC article.
-
HIV-1 RRE RNA acts as an RNA silencing suppressor by competing with TRBP-bound siRNAs.RNA Biol. 2015;12(2):123-35. doi: 10.1080/15476286.2015.1014759. RNA Biol. 2015. PMID: 25668122 Free PMC article.
-
Distinguishable in vitro binding mode of monomeric TRBP and dimeric PACT with siRNA.PLoS One. 2013 May 2;8(5):e63434. doi: 10.1371/journal.pone.0063434. Print 2013. PLoS One. 2013. PMID: 23658827 Free PMC article.
-
Intracellular localization and routing of miRNA and RNAi pathway components.Curr Top Med Chem. 2012;12(2):79-88. doi: 10.2174/156802612798919132. Curr Top Med Chem. 2012. PMID: 22196276 Review.
-
[Components and assembly of RNA-induced silencing complex].Yi Chuan. 2006 Jun;28(6):761-6. Yi Chuan. 2006. PMID: 16818443 Review. Chinese.
Cited by
-
Calcium phosphate nanoparticles-based systems for siRNA delivery.Regen Biomater. 2016 Sep;3(3):187-95. doi: 10.1093/rb/rbw010. Epub 2016 Mar 4. Regen Biomater. 2016. PMID: 27252888 Free PMC article. Review.
-
The siRNA Non-seed Region and Its Target Sequences Are Auxiliary Determinants of Off-Target Effects.PLoS Comput Biol. 2015 Dec 11;11(12):e1004656. doi: 10.1371/journal.pcbi.1004656. eCollection 2015 Dec. PLoS Comput Biol. 2015. PMID: 26657993 Free PMC article.
-
Characterization of Argonaute-containing protein complexes in Leishmania-infected human macrophages.PLoS One. 2024 May 23;19(5):e0303686. doi: 10.1371/journal.pone.0303686. eCollection 2024. PLoS One. 2024. PMID: 38781128 Free PMC article.
-
Utilizing Selected Di- and Trinucleotides of siRNA to Predict RNAi Activity.Comput Math Methods Med. 2017;2017:5043984. doi: 10.1155/2017/5043984. Epub 2017 Jan 24. Comput Math Methods Med. 2017. PMID: 28243313 Free PMC article.
-
Expression of TARBP1 protein in human non-small-cell lung cancer and its prognostic significance.Oncol Lett. 2018 May;15(5):7182-7190. doi: 10.3892/ol.2018.8202. Epub 2018 Mar 7. Oncol Lett. 2018. PMID: 29731880 Free PMC article.
References
-
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. - PubMed
-
- Novia CD, Sharp PA. The RNAi revolution. Nature. 2004;430:161–164. - PubMed
-
- Stark GR, Kerr IM, Williams BRG, Silverman RH, Schreiber RD. How cells respond to interferons. Biochemistry. 1998;67:227–264. - PubMed
-
- Clemens MJ, Elia A. The double-stranded RNA-dependent protein kinase PKR: structure and function. J. Interferon Cytokine Res. 1997;17:503–524. - PubMed
-
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

