Mechanical control of the sense of touch by β-spectrin
- PMID: 24561618
- PMCID: PMC4046587
- DOI: 10.1038/ncb2915
Mechanical control of the sense of touch by β-spectrin
Abstract
The ability to sense and respond to mechanical stimuli emanates from sensory neurons and is shared by most, if not all, animals. Exactly how such neurons receive and distribute mechanical signals during touch sensation remains mysterious. Here, we show that sensation of mechanical forces depends on a continuous, pre-stressed spectrin cytoskeleton inside neurons. Mutations in the tetramerization domain of Caenorhabditis elegans β-spectrin (UNC-70), an actin-membrane crosslinker, cause defects in sensory neuron morphology under compressive stress in moving animals. Through atomic force spectroscopy experiments on isolated neurons, in vivo laser axotomy and fluorescence resonance energy transfer imaging to measure force across single cells and molecules, we show that spectrin is held under constitutive tension in living animals, which contributes to elevated pre-stress in touch receptor neurons. Genetic manipulations that decrease such spectrin-dependent tension also selectively impair touch sensation, suggesting that such pre-tension is essential for efficient responses to external mechanical stimuli.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
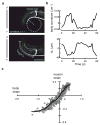
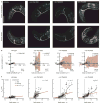

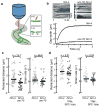

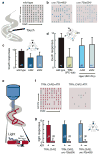
Similar articles
-
The tactile receptive fields of freely moving Caenorhabditis elegans nematodes.Integr Biol (Camb). 2018 Aug 1;10(8):450-463. doi: 10.1039/c8ib00045j. Epub 2018 Jul 20. Integr Biol (Camb). 2018. PMID: 30027970 Free PMC article.
-
Mechanical systems biology of C. elegans touch sensation.Bioessays. 2015 Mar;37(3):335-44. doi: 10.1002/bies.201400154. Epub 2015 Jan 19. Bioessays. 2015. PMID: 25597279 Free PMC article. Review.
-
Touch-induced mechanical strain in somatosensory neurons is independent of extracellular matrix mutations in Caenorhabditis elegans.Mol Biol Cell. 2020 Jul 21;31(16):1735-1743. doi: 10.1091/mbc.E20-01-0049. Epub 2020 Jun 24. Mol Biol Cell. 2020. PMID: 32579427 Free PMC article.
-
The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals.Nat Neurosci. 2005 Jan;8(1):43-50. doi: 10.1038/nn1362. Epub 2004 Dec 5. Nat Neurosci. 2005. PMID: 15580270
-
The cell biology of touch.J Cell Biol. 2010 Oct 18;191(2):237-48. doi: 10.1083/jcb.201006074. J Cell Biol. 2010. PMID: 20956378 Free PMC article. Review.
Cited by
-
Identification of ceftazidime interaction with bacteria in wastewater treatment by Raman spectroscopic mapping.RSC Adv. 2019 Oct 15;9(56):32744-32752. doi: 10.1039/c9ra06006e. eCollection 2019 Oct 10. RSC Adv. 2019. PMID: 35529746 Free PMC article.
-
Epidermal control of axonal attachment via β-spectrin and the GTPase-activating protein TBC-10 prevents axonal degeneration.Nat Commun. 2020 Jan 9;11(1):133. doi: 10.1038/s41467-019-13795-x. Nat Commun. 2020. PMID: 31919407 Free PMC article.
-
Structural plasticity of actin-spectrin membrane skeleton and functional role of actin and spectrin in axon degeneration.Elife. 2019 May 1;8:e38730. doi: 10.7554/eLife.38730. Elife. 2019. PMID: 31042147 Free PMC article.
-
HMP-1/α-catenin promotes junctional mechanical integrity during morphogenesis.PLoS One. 2018 Feb 21;13(2):e0193279. doi: 10.1371/journal.pone.0193279. eCollection 2018. PLoS One. 2018. PMID: 29466456 Free PMC article.
-
Pushing, pulling, and squeezing our way to understanding mechanotransduction.Methods. 2016 Feb 1;94:4-12. doi: 10.1016/j.ymeth.2015.08.019. Epub 2015 Aug 28. Methods. 2016. PMID: 26318086 Free PMC article. Review.
References
-
- O’Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nature Neuroscience. 2005;8:43–50. - PubMed
-
- Suzuki H, et al. in vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron. 2003;39:1005–1017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

