N-Propargylated isatin-Mannich mono- and bis-adducts: synthesis and preliminary analysis of in vitro activity against Tritrichomonas foetus
- PMID: 24561663
- PMCID: PMC7115568
- DOI: 10.1016/j.ejmech.2014.01.015
N-Propargylated isatin-Mannich mono- and bis-adducts: synthesis and preliminary analysis of in vitro activity against Tritrichomonas foetus
Abstract
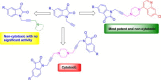
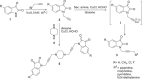
Cu(I)Cl promoted synthesis of N-propargylated-isatin Mannich mono- and bis-adducts with an extension towards the synthesis of N-propargylated-isatin-7-chloroquinoline conjugates was described. The synthesized scaffolds were evaluated for their in vitro activity against the veterinary protozoal pathogen Tritrichomonas foetus and cytotoxicity against human prostate (PC-3) cancer cell line. The preliminary evaluation data revealed the enhancement in the activity profiles with the introduction of 7-chloroquinoline ring with the most active conjugates 7a, 7c and 7d exhibiting an IC₅₀ of 22.2, 11.3 and 24.5 μM respectively against T. foetus and minimal toxicity against human prostate (PC-3) cell lines.
Keywords: Cytotoxicity; N-Propargylated-isatin Mannich adducts; N-Propargylated-isatin-7-chloroquinoline conjugates; Tritrichomonas foetus.
Copyright © 2014 Elsevier Masson SAS. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous